

|
Author(s)
Max Troxler
Kathryn Vowden
Peter Vowden
|
Contents
|
|
Published:
December 2006
Last updated: December 2006 Revision: 1.0 |
Keywords: chronic wounds; hard-to-heal wounds; wound healing; wound assessment; wound measurement; wound duration; ischaemia.
Some wounds do not heal for several years and can be defined as ‘hard to heal’.
Predicting healing potential at assessment is essential if cost-effective and clinically effective care is to be given.
Ulcer size and duration, and the initial response to treatment, can be used as predictors of healing.
Simple assessment techniques can identify wounds that may be hard to heal and ensure that adjuvant therapies and expensive treatment options are used appropriately.
It is important that treatment decisions for patients with chronic wounds result in a cost-effective and clinically effective allocation of healthcare resources. Predicting the healing potential of a wound is an essential part of such decision-making, and need not be a complicated process. A number of wound and patient characteristics that may affect the outcome of treatment in the case of venous and diabetic wounds have been identified, and should be considered at the initial patient assessment. Relatively simple techniques for measuring ulcer size can be used, along with information about the duration of the ulcer, to predict the success of standard treatments. The initial response to treatment is also a predictive factor. Identification of those wounds that are likely to be 'hard to heal' enables the aggressive use of adjunctive therapies to be targeted at those patients who need it.
A considerable proportion of healthcare resources is spent on the management of patients with chronic wounds [1] [2] [3]. Venous leg ulcers, diabetic foot ulcers and pressure ulcers account for more than 90% of these wounds [4], and although healing often follows a predictable course, some may remain unhealed for many years. A 'hard-to-heal' wound can be defined as one that fails to heal with 'standard therapy' in an orderly and timely manner. The complex pathophysiology of chronic wounds and impaired healing is beginning to be understood, and the challenge is to use this understanding to achieve cost-effective care. When assessing a new patient, the consideration of factors that help in the prompt identification of 'hard-to-heal' wounds will allow early aggressive application of adjunctive therapies, thus helping to maximise treatment success and avoid costly inadequate interventions, clinician frustration and patient disillusionment.
The healing of acute wounds has traditionally been divided into three overlapping phases: inflammation, proliferation and remodelling. The first involves haemostasis and the recruitment of neutrophils to destroy bacteria and necrotic tissue, followed by macrophages, whose presence is important for the transition to the proliferative phase [5]. This second phase involves activation of fibroblasts and endothelial cells to produce granulation tissue, together with attempted re-epithelialisation. During the remodelling phase the size and orientation of deposited collagen within the healed wound is slowly altered to achieve maximal strength [6].
In contrast with the acute situation, in hard-to-heal wounds the highly orchestrated reparative response breaks down. In diabetic wounds, for example, the recruitment of inflammatory cells is impaired, allowing time for infection to become established [7]. In chronic wounds, there is a tendency for the inflammatory response, which is initially so important, to become exaggerated. Increased infiltration of inflammatory cells, greater secretion of proinflammatory cytokine, elevated generation of reactive oxygen species and increased production of proteolytic enzymes are combined with reduced inhibitor release, for example tissue inhibitors of metalloproteinases (TIMPs) [8] [9]. Elevated expression of the cytokines, tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 upregulates the synthesis of several matrix metalloproteinases (MMP-1, MMP-2, MMP-8, MMP-9) and serine proteases (elastase, plasmin) which, in excess, cause not only deleterious extracellular matrix destruction but also growth factor inactivation [8] [10] [11] [12]. The chronic wound environment therefore consists of sustained matrix degradation, reduced growth factor bioavailability and increased fibroblast senescence, which all combine to inhibit cellular proliferation, angiogenesis and tissue repair.
Predicting healing potential in individual patients is an essential part of management if cost-effective and clinically effective care is to be given, although this need not be a complex process. A number of wound and patient characteristics that may delay healing of both acute and chronic wounds and predispose wounds to recurrence have been defined (Table 1). The levels of some biological factors have also been related to subsequent wound healing. These include markers of collagen remodelling in venous ulcers and ratios of MMP-9/TIMP-1 in pressure ulcers [13] [14].
| Factors |
| Age |
| Anaemia |
| Decreased oxygen |
| Decreased perfusion |
| Dehydration |
| Diabetes mellitus |
| Foreign body |
| Malignancy |
| Medication, such as corticosteroids, cytotoxics |
| Immunosuppression |
| Microbes |
| Necrotic tissue |
| Nutrition (vitamins, minerals) |
| Oedema |
| Organ failure |
| Radiation |
| Smoking |
| Other wound-related factors |
Wound healing encompasses a series of complex processes that involve different cell types, growth factors and extracellular matrix (ECM) components. Unless these biological processes are interrupted, these three key players together contribute to the healed wound.
It has been known for many years that other ECM components, in addition to collagen, dominate the wound healing process. More recent research has also revealed the biological properties of these wound-specific components of the ECM. For example, it has been discovered that in order for a cell, such as a fibroblast, to respond to a growth factor it needs to adhere to the ECM. These cell-ECM contacts govern many cell functions such as the synthesis of growth factors and the production of new ECM during wound healing. The ECM composition is also a very important determinant of the formation of blood vessels in a wound. Thus, by controlling the structure and composition of the ECM, it is possible to manipulate the wound healing process [15].
High compression bandaging is now recognised as the main element in the treatment of venous leg ulceration, yet the majority of published clinical data indicate that only 65% of ulcers are likely to be healed within 24 weeks of appropriate compression therapy, with 20% of ulcers remaining unhealed after more than 50 weeks [16]. Skene et al, in a randomised study involving 200 patients, found that, in the presence of graduated compression, healing occurred more rapidly in patients with a smaller ulcer area (RR 1.92), shorter duration of ulceration (RR 1.35), younger age (RR 1.34) and no deep vein involvement (RR 1.8) [17].
Margolis et al, in a retrospective cohort study of 260 patients, identified that large wound area, duration of the wound in months, a history of venous ligation or venous stripping, a history of hip or knee replacement surgery, an ankle-brachial index below 0.80, and the presence of fibrin on more than 50% of the wound surface were significantly associated with delayed wound healing at 24 weeks [18]. Others have commented on additional factors, such as patient and/or ankle mobility, body mass index, age, compliance, deep vein involvement, gender and diabetes, as well as social and economic factors, such as living alone or the availability of central heating [19] [20] [21]. Many of these can be identified at the first patient assessment and should therefore be addressed as part of the initial treatment plan.
If a more simple assessment of healing potential is required, a specific relationship between ulcer size and duration and the likelihood of healing has been defined. Margolis et al were able to demonstrate that a scoring system related to ulcer size (>5cm2 = 1 point) and duration (>6 months = 1 point) gave a good indication of likely outcome, with 93% of ulcers with a score of 0 healing at 24 weeks as compared to only 37% of those scoring 2 [22]. Although it may seem a self-fulfilling prophecy that a long ulcer duration predicts poor response to standard therapy, appreciation of this concept is vital if appropriate intervention is to be applied and a realistic prognosis given.
Steed et al have demonstrated the differing, and early separation of, healing trajectories of ulcers that subsequently heal and those that remain unhealed after up to 20 weeks of care [23]. Prince and Dodds have shown that venous ulcers that respond to treatment do so at an almost constant rate and that the initial response to treatment can be a reliable predictor of estimated healing time [24]. These observations support the earlier findings of Phillips et al and van Rijswijk, who found that the early response of a venous leg ulcer to appropriate care was highly suggestive of subsequent healing times [25] [26]. These systems are based on Gilman’s formula (Figure 1), which attempts to compensate for variations in ulcer size and shape at onset, and appear to give a good prediction of healing based on early response to treatment [27].

Phillips et al, looking at percentage reduction in ulcer area, found that approximately 77% of outcomes could be predicted based on a size reduction of more than 44% at three weeks [25]. Likewise, van Rijswijk suggested that a reduction in ulcer area greater than 30% as early as two weeks was predictive of outcome [26]. Gelfand et al, in an analysis of 29,189 patients, confirmed that, based on the area under the receiver operator characteristic curve log rate, log area ratio and percentage change in area can indicate which patients will heal at 12 or 24 weeks of care (receiver operator characteristic 0.72-0.80) [28].
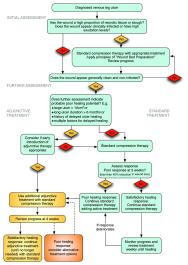
All of these observations indicate that, with careful assessment and repeated wound measurement - which must be accurate – it should be possible to identify a sub-population of patients with hard-to-heal venous ulcers within the first few weeks of treatment [29] [30]. As well as allowing the targeting of adjuvant therapies, methods of tracking wound healing also allow healthcare professionals to give patients an indication of likely treatment times, and facilitate the identification of complications, such as infection, at an earlier stage [24]. Figure 2 indicates how adjunctive therapy could be included in a management algorithm based on both the assessment of initial risk factors and the evaluation of treatment response to standard care.
Hareendran et al demonstrated that Skindex scores indicated that older patients had worse health-related quality of life (p<0.05), as did those with pain and non-healing ulcers [31]. Several phenomenological studies have confirmed the impact of non-healing ulceration on patients, focusing on coping strategies and life disruption [32] [33].The use of appropriate treatment strategies can aid healing, improve quality of life and patient adherence with treatment [34].
The aetiology of lower limb ulceration in patients with diabetes mellitus varies according to the exact population studied but can be classified as neuropathic (50-67%), arterial (1-20%) or neuroischaemic (26-30%) [35] [36] [37] [38].
Margolis et al retrospectively studied 27,630 patients with diabetic neuropathic ulcers from a wound care database spanning 18 years and encompassing 150 treatment centres [38]. Standard therapy for neuropathic ulcers was employed, involving wound debridement, moist dressings and offloading techniques. They concluded that size (>2cm2), duration (>2 months) and depth of the ulcer (penetrating through skin to expose tendon, ligament or joint, or worse) were the three most important prognostic factors. Those wounds displaying none of these factors had a 66% probability of healing by 20 weeks, compared to only 22% for those with all three criteria.
In a transatlantic collaboration, Oyibo et al studied a group of 194 patients, 67% of whom had neuropathic and 26% neuroischaemic foot ulcers [35]. After 26 weeks of follow-up, only 65% had healed completely and 16% had required an amputation, of which 70% were distal to the mid-foot. Interestingly, age, sex, ulcer site, diabetes type and diabetes duration had no predictive effect on wound healing. Ulcer area at presentation, however, was predictive, but only to a moderate degree (hazard ratio 1.08, 95% CI 1.01-1.14). However, the presence of ischaemia had a profound influence on healing; it doubled the time taken to heal and tripled the risk of amputation.
Zimny et al also found the presence of ischaemia to have a marked effect on foot ulcer healing [39]. They found that the healing rate in patients with neuropathic ulcers, expressed as a reduction in wound radius, was 2.4 times faster than that of patients with neuroischaemic wounds, and seven times faster than those with arterial ulcers. In patients with neuropathic and neuroischaemic foot ulcers, the initial size of the wound correlated strongly with the time taken to heal, but this was not the case with purely ischaemic ulceration. This reinforces the importance of a thorough assessment of lower limb perfusion. Arterial calcification may lead to a falsely reassuring ankle-brachial pressure index but toe pressures or transcutaneous oxygen tension measurements are more accurate in patients with diabetes. Toe pressure below 30mmHg, and/or transcutaneous oxygen tension lower than 25mmHg, predict poor healing [40] [41]. Therefore, if significant ischaemia is found, referral to a vascular surgeon for consideration of revascularisation is highly recommended.
There is a general belief that 'heel ulcers do not heal' but recent studies suggest that this is not the case. Both Apelqvist and Oyibo have found that the site of foot ulceration is unassociated with outcome [42] [35]. Similarly, Chipchase et al, in a study of 97 diabetic patients with heel ulcers and a preference for conservative treatment, found that 66% of ulcers did eventually heal (median 200 days) albeit more slowly than an unselected population of foot ulcers (median 126 days) [43]. Consistent with the prognostic factors cited above, the ulcer area and the presence of ischaemia were the two most important predictors of successful outcome.
Several classification systems for diabetic foot ulceration have been devised to allow like-to-like comparison of wounds. Those requiring the determination of the presence of infection, ischaemia and neuropathy, in that order, have been advocated because they reflect the order in which clinical decisions should be made [36] [44]. One such example is the S(AD) SAD or 'size (area and depth), sepsis, arteriopathy and denervation’ classification system [45]. This was validated in a cohort of 300 patients and logistic regression analysis again determined that the most important factors associated with completed healing were the ulcer size (expressed as depth and area) and the presence of arteriopathy.
As with venous leg ulceration, the response to initial treatment has been shown to predict outcome. Sheehan et al prospectively studied 276 patients with foot ulcers that showed no evidence of infection or ischaemia and did not penetrate to bone or tendon [46]. Treatments consisted of wound debridement and offloading techniques and the study compared moist gauze with a collagen/oxidised regenerated cellulose dressing (Promogran®). Changes in wound area were compared, measured simply by multiplying length and width, and it was found that a reduction in size of more than 53% at four weeks of treatment predicted complete healing in 58% of cases by 12 weeks. In patients whose ulcer area reduced by ≤53% at four weeks, complete healing was achieved in only 9% (sensitivity 91%, negative predictive value 91%). This predictive threshold was independent of treatment modality. The mean percentage reduction in ulcer area during the first four weeks was 82% in the Promogran group and 82% in the control patients who went on to heal. Likewise, the mean percentage reduction in ulcer area during the first four weeks was 25% in both the Promogran and control patients who did not heal.
Similarly, Zimny et al determined the wound radius reduction rate by estimating the wound area (multiplying the two longest diameters) and then calculating the radius of the wound (see Figure 3) and plotting changes over time [39]. Their findings indicate that the healing of neuropathic foot ulcers, with no evidence of osteomyelitis, can be predicted with reasonable reliability through the measurement of wound radius reduction rate that displays a linear correlation with time [47] [48]. They found that all neuropathic foot ulcers showing no evidence of osteomyelitis healed eventually and that, whatever the starting size of the ulcer, the wound radius reduced in a reasonably linear fashion over time. As with venous leg ulcers, these methods for tracking diabetic ulcer healing can be used to guide the use of adjunctive therapy, identify complications and provide an indication of treatment times.

There is a wealth of reports documenting the many factors that predispose to the development of pressure ulcers [2] [49]. In addition, wound scoring systems such as DESIGN and PUSH can score a wound and monitor healing [50] [51] [52]. In contrast to venous and diabetic ulcers, there is a paucity of literature on pressure ulcers related to the factors that predict healing. However, the factors that predict ‘hard-to-heal’ venous leg and diabetic foot ulcers are likely to be important in this type of chronic wound, although the assessment of wound size may be difficult. Certain factors, however, have been associated with wound healing and these include a lower ulcer grade and greater patient weight [53] [54]. The comparatively weak correlation between ulcer grade and healing may be accounted for by variable tissue sensitivity to pressure, friction and shear. Fat is more resistant than skin and muscle, resulting in hourglass shaped wounds, where the degree of tissue injury is underestimated by superficial inspection alone. The positive correlation with body weight may at first seem counter-intuitive but may reflect better healing responses due to improved nutrition. Conversely, other factors are associated with failure to heal and these include remaining chair/bed bound and the presence of faecal incontinence [55] [56].
It is now possible for the clinician, when assessing a patient who has a venous or diabetic ulcer, to predict with some reliability, using relatively simple techniques, which wounds are likely to be hard to heal. Increased understanding of the wound environment will provide opportunities to manipulate the wound bed to promote more rapid and potentially improved quality of healing for these patients. Adjuvant therapies may include pharmacotherapy (horse-chestnut extract), advanced wound care products (growth factors, interactive dressings, for example Xelma™, Promogran®), physical therapies (intermittent pneumatic compression, topical negative pressure therapy), biological therapy (larvae) and surgery (venous surgery, grafting, joint excision) [16] [57] [58] [59] [60] [61] [62] [63] [64] [65] [66].
Such therapeutic options are likely to be significantly more expensive than conventional dressings, however. For these products the key to their widespread adoption will be the identification of specific target populations in whom cost-effectiveness can be demonstrated due to a reduction in healing time and/or a reduction in required healthcare professional time. The introduction of an adjuvant therapy should not, however, be seen as an alternative to standard care as defined by national and international management guidelines, appropriate holistic care or the application of wound bed preparation techniques.
1. Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg 2006; 117(7 Suppl): 193S-209S; discussion 210S-211S.
2. Niezgoda JA, Mendez-Eastman S. The effective management of pressure ulcers. Adv Skin Wound Care 2006; 19(Suppl 1): 3-15.
3. Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg 2004; 188(1A Suppl): 1-8.
4. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 2006; 117(7 Suppl): 35S-41S.
5. Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am 1997; 77(3): 509-28.
6. Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg 2006; 117(7 Suppl): 1e-S-32e-S.
7. Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol 2001; 159(2): 513-25.
8. Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil 2005; 26(4): 306-19.
9. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999; 7(6): 442-52.
10. Harding KG, Morris HL, Patel GK. Science, medicine and the future: healing chronic wounds. BMJ 2002; 324(7330): 160-3.
11. Lauer G, Sollberg S, Cole M, Flamme I, Sturzebecher J, Mann K, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol 2000; 115(1): 12-18.
12. Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997; 5(1): 23-32.
13. Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regen 1999; 7(5): 347-55.
14. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002; 10(1): 26-37.
15. Schultz GS, Ladwig G, Wysocki A. Extracellular matrix: review of its roles in acute and chronic wounds. World Wide Wounds (online) 2005; available from URL: http://www.worldwidewounds.com/2005/august/Schultz/Extrace-Matric-Acute-Chronic-Wounds.html.
16. Barwell JR, Davies CE, Deacon J, Harvey K, Minor J, Sassano A, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet 2004; 363(9424): 1854-9.
17. Skene AI, Smith JM, Dore CJ, Charlett A, Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ 1992; 305(6862): 1119-21.
18. Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 1999; 135(8): 920-6.
19. Franks PJ, Bosanquet N, Connolly M, Oldroyd MI, Moffatt CJ, Greenhalgh RM, et al. Venous ulcer healing: effect of socioeconomic factors in London. J Epidemiol Community Health 1995; 49(4): 385-8.
20. Franks PJ, Moffatt CJ, Connolly M, Bosanquet N, Oldroyd MI, Greenhalgh RM, et al. Factors associated with healing leg ulceration with high compression. Age Ageing 1995; 24(5): 407-10.
21. Meaume S, Couilliet D, Vin F. Prognostic factors for venous ulcer healing in a non-selected population of ambulatory patients. J Wound Care 2005; 14(1): 31-4.
22. Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004; 12(2): 163-8.
23. Steed DL, Hill DP, Woodske ME, Payne WG, Robson MC. Wound-healing trajectories as outcome measures of venous stasis ulcer treatment. Int Wound J 2006; 3(1): 40-7.
24. Prince S, Dodds SR. Use of ulcer size and initial responses to treatment to predict the healing time of leg ulcers. J Wound Care 2006; 15(7): 299-303.
25. Phillips TJ, Machado F, Trout R, Porter J, Olin J, Falanga V. Prognostic indicators in venous ulcers. J Am Acad Dermatol 2000; 43(4): 627-30.
26. van Rijswijk L. Full-thickness leg ulcers: patient demographics and predictors of healing. Multi-Center Leg Ulcer Study Group. J Fam Pract 1993; 36(6): 625-32.
27. Gilman T. Wound outcomes: the utility of surface measures. Int J Low Extrem Wounds 2004; 3(3): 125-32.
28. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002; 119(6): 1420-5.
29. Flanagan M. Improving accuracy of wound measurement in clinical practice. Ostomy Wound Manage 2003; 49(10): 28-40.
30. Flanagan M. Wound measurement: can it help us to monitor progression to healing? J Wound Care 2003; 12(5): 189-94.
31. Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, et al. Measuring the impact of venous leg ulcers on quality of life. J Wound Care 2005; 14(2): 53-7.
32. Hopkins A. Disrupted lives: investigating coping strategies for non-healing leg ulcers. Br J Nurs 2004; 13(9): 556-63.
33. Beitz JM, Goldberg E. The lived experience of having a chronic wound: a phenomenologic study. Medsurg Nurs 2005; 14(1): 51-62, 82.
34. Marston W, Vowden K. Compression therapy: a guide to safe practice. In: EWMA Position Document. Understanding compression therapy. London: MEP Ltd, 2003; 11-17.
35. Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB, et al. The effects of ulcer size and site, patient's age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med 2001; 18(2): 133-8.
36. Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet 2005; 366(9498): 1725-35.
37. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005; 366(9498): 1736-43.
38. Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med 2003; 115(8): 627-31.
39. Zimny S, Schatz H, Pfohl M. Determinants and estimation of healing times in diabetic foot ulcers. J Diabetes Complications 2002; 16(5): 327-32.
40. Wutschert R, Bounameaux H. Predicting healing of arterial leg ulcers by means of segmental systolic pressure measurements. Vasa 1998; 27(4): 224-8.
41. Kalani M, Brismar K, Fagrell B, Ostergren J, Jorneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999; 22(1): 147-51.
42. Apelqvist J, Castenfors J, Larsson J, Stenstrom A, Agardh CD. Wound classification is more important than site of ulceration in the outcome of diabetic foot ulcers. Diabet Med 1989; 6(6): 526-30.
43. Chipchase SY, Treece KA, Pound N, Game FL, Jeffcoate WJ. Heel ulcers don't heal in diabetes. Or do they? Diabet Med 2005; 22(9): 1258-62.
44. Jeffcoate WJ, Macfarlane RM, Fletcher EM. The description and classification of diabetic foot lesions. Diabet Med 1993; 10(7): 676-9.
45. Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ. Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med 2004; 21(9): 987-91.
46. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003; 26(6): 1879-82.
47. Zimny S, Pfohl M. Healing times and prediction of wound healing in neuropathic diabetic foot ulcers: a prospective study. Exp Clin Endocrinol Diabetes 2005; 113(2): 90-3.
48. Zimny S, Schatz H, Pfohl M. The effects of ulcer size on the wound radius reductions and healing times in neuropathic diabetic foot ulcers. Exp Clin Endocrinol Diabetes 2004; 112(4): 191-4.
49. Brem H, Lyder C. Protocol for the successful treatment of pressure ulcers. Am J Surg 2004; 188(1A Suppl): 9-17.
50. Sanada H, Moriguchi T, Miyachi Y, Ohura T, Nakajo T, Tokunaga K, et al. Reliability and validity of DESIGN, a tool that classifies pressure ulcer severity and monitors healing. J Wound Care 2004; 13(1): 13-8.
51. Stotts NA, Rodeheaver GT, Thomas DR, Frantz RA, Bartolucci AA, Sussman C, et al. An instrument to measure healing in pressure ulcers: development and validation of the pressure ulcer scale for healing (PUSH). J Gerontol A Biol Sci Med Sci 2001; 56(12): M795-9.
52. Thomas DR, Rodeheaver GT, Bartolucci AA, Franz RA, Sussman C, Ferrell BA, et al. Pressure ulcer scale for healing: derivation and validation of the PUSH tool. The PUSH Task Force. Adv Wound Care 1997; 10(5): 96-101.
53. Kramer JD, Kearney M. Patient, wound, and treatment characteristics associated with healing in pressure ulcers. Adv Skin Wound Care 2000; 13(1): 17-24.
54. Itoh M, Montemayor JS, Matsumoto E, Eason A, Lee MH, Folk FS. Accelerated wound healing of pressure ulcers by pulsed high peak power electromagnetic energy (Diapulse). Decubitus 1991; 4(1): 24-5, 29-34.
55. Berlowitz DR, Wilking SV. The short-term outcome of pressure sores. J Am Geriatr Soc 1990; 38(7): 748-52.
56. Ferrell BA, Osterweil D, Christenson P. A randomized trial of low-air-loss beds for treatment of pressure ulcers. JAMA 1993; 269(4): 494-7.
57. Leach MJ, Pincombe J, Foster G. Clinical efficacy of horsechestnut seed extract in the treatment of venous ulceration. J Wound Care 2006; 15(4): 159-67.
58. Suter A, Bommer S, Rechner J. Treatment of patients with venous insufficiency with fresh plant horse chestnut seed extract: a review of 5 clinical studies. Adv Ther 2006; 23(1): 179-90.
59. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 1998; 21(5): 822-7.
60. Omar AA, Mavor AI, Jones AM, Homer-Vanniasinkam S. Treatment of venous leg ulcers with Dermagraft. Eur J Vasc Endovasc Surg 2004; 27(6): 666-72.
61. Cavorsi J, Vicari F, Wirthlin DJ, Ennis W, Kirsner R, O'Connell SM, et al. Best-practice algorithms for the use of a bilayered living cell therapy (Apligraf) in the treatment of lower-extremity ulcers. Wound Repair Regen 2006; 14(2): 102-9.
62. Vowden K. The use of intermittent pneumatic compression in venous ulceration. Br J Nurs 2001; 10(8): 491-509.
63. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) device: a review. Am J Clin Dermatol 2005; 6(3): 185-94.
64. Raynor P, Dumville J, Cullum N. A new clinical trial of the effect of larval therapy. J Tissue Viability 2004; 14: 104-5.
65. Jones JE, Nelson EA. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev 2000; (2): CD001737.
66. Griffiths GD, Wieman TJ. Metatarsal head resection for diabetic foot ulcers. Arch Surg 1990; 125(7): 832-5.