

|
Author(s)
Mieke Flour
|
Contents
|
|
Published:
September 2009
Last updated: September 2009 Revision: 1.0 |
Keywords: normal skin; vulnerable skin; skin barrier function; ageing skin.
Skin is a dynamic organ that is continuously renewing and altering itself in response to endogenous and exogenous stimuli. These processes can malfunction in people with vulnerable skin.
Ageing, UV radiation damage and a genetic predisposition all contribute to skin vulnerability.
Some factors that contribute to the problem of vulnerable skin could be prevented, such as the use of irritants, stripping by adhesive dressings, occlusion and exposure to infection or allergens.
Healthcare professionals should be aware of the problems that vulnerable skin may cause in patients with wounds.
This is the first in a series of three articles examining the causes and consequences of vulnerable skin. This article describes the physiology of normal skin and examines the causes, both intrinsic and extrinsic, of skin vulnerability. Subsequent articles will examine the problems that may arise in vulnerable periwound skin and the steps healthcare professionals can take to ameliorate them.
Normal skin (see Figure 1) fulfils many functions, a primary one being to protect the body against chemical, physical and mechanical hazards and invasions by micro-organisms [1]. The skin contains several types of sensory receptors that detect the incoming stimuli of touch, pain, vibration, pressure, warmth, cold and itch.
The efficacy of the skin barrier function is determined by a number of characteristics. These include the quality of the epidermal cells, of the interaction between cells, of the epidermis and dermis, of the intercellular substances, and of the immune system. Skin is a dynamic organ that is continuously renewing and altering itself in response to endogenous and exogenous stimuli.
To gain an understanding of the altered physiology that leads to skin becoming vulnerable, it is necessary to first understand the physiology of healthy skin.
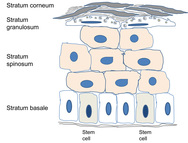
The process of epidermal renewal is ensured by antigen p63 and CD29 (integrin ß-1B), expressed in epidermal stem cells in the basal layer. These proliferative progenitor cells have the capacity for self-renewal and for generating the differentiated cells that offer a better resistance to environmental hazards (see Figure 2). By developing a stratified epithelium of terminally differentiating cells, the epidermis generates a self-perpetuating barrier that helps keep harmful micro-organisms out and essential body fluids in. These stem cells reside in the adult hair follicles, sebaceous glands and epidermis.
Transcription factor p63 is implicated in the maintenance of epithelial stem cells in stratified epithelia. It regulates the expression of extracellular matrix adhesion molecules, including basal integrins, which are necessary to orient asymmetric cell divisions during epithelial stratification, and desmosomal proteins, which are determinants of epithelial integrity and proliferative maintenance [2]. MicroRNA 203 stops the translation and suppresses the activity of p63, which results in a transition from proliferating stem cells to differentiating keratinocytes as they exit the basal layer and move outward to the skin surface. CD29 is a type 1 glycoprotein expressed on most cells, of which the isoform, known as integrin ß-1B, is expressed by all basal keratinocytes in the skin. It binds to several cell surfaces, acts as a fibronectin receptor and has adhesion to ligands such as matrix proteins, collagen and laminin. Integrin heterodimers containing CD29 mediate cell-to-cell and cell-to-matrix adhesion, and are also involved in a variety of processes including embryogenesis, tissue repair and development. Basal keratinocytes express CD29, which maintains them in an undifferentiated state; its expression is downregulated in keratinocytes that have left the basal layer and initiated the differentiation programme.
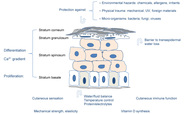
The suprabasal keratinocytes differentiate into a chemically and physically resistant horny layer surrounded by proteins and lipids, including ceramides, cholesterol and fatty acids. Natural or forced removal of the top layers of this cornified epithelium will stimulate turnover by the underlying cells to replace the damaged or lost cells. This cornified layer provides the protective and water-barrier functions between the body and the environment [3].
Low calcium concentrations in the basal proliferating layer, and a progressively higher concentration as one proceeds to the outer differentiated layers, constitute the so-called epidermal calcium gradient (see Figure 2). Assembly of the cornified envelope is precisely regulated and triggered by this Ca2+ gradient coincident with cellular signals and processes of terminal keratinocyte differentiation.
The acidic pH of the horny layer is called the ‘acid mantle’ of the stratum corneum, and is important for both cutaneous antimicrobial defence and the formation of a barrier against permeability. The pH of the skin follows a sharp gradient across the horny layer, controlling activities of pH-dependent enzymes, which regulate skin cornification, desquamation and homeostasis of the barrier function. The lamellar extracellular arrangement of barrier lipids requires an acidic milieu. Normal pH on the surface of adult skin is in the range of 5.4 to 5.9, due to the components of the stratum corneum, sebum and sweat secretion. Endogenous and exogenous influences determine the acidity of the skin: most important are age, anatomic site, the use of detergents (soaps or synthetic detergents) and cosmetic products, occlusion by body folds or dressings, skin irritants and the use of topical pharmacological substances [4].
In the very young child the skin pH is typically slightly acid at 5.5. This usually remains constant in adulthood, and increases to be more alkaline in elderly people. Moist body folds, the axillae, genito-anal region and the interdigital spaces have a slightly more alkaline pH and are more prone to yeast and fungal infections. Following the use of soap the pH is raised for a few hours; this is much less pronounced if acidic synthetic detergents are used. The pH can rise significantly under occlusive dressings and on exposure to skin irritants such as ammonia and stool enzymes in nappies or continence pads, resulting in disruption of the skin barrier and irritation [5].
Changes in skin pH may play a significant role in the pathogenesis, prevention and treatment of irritant contact dermatitis, atopic dermatitis, ichthyosis, acne vulgaris, Candida albicans infections and wound healing. The acidity of the skin surface is said to be bacteriostatic for some strains: most bacteria grow better in a neutral pH. A correlation between pH and bacterial growth has been described for propionibacteria after the use of alkaline soap on the forehead [5] and for the development of mycoses in skin folds in patients with diabetes and patients on dialysis [6].
The epidermis has a degree of mechanical strength to withstand damage, and the ability to repair itself if injured. The dermis provides elasticity in response to mechanical stress. The permeability of the skin depends on the presence of chemical substances in the stratum corneum, the viable epidermis and the uppermost layer of the dermis. The efficiency of this barrier varies between body sites: the scrotum is particularly permeable, while the forehead and the dorsa of the hands are more permeable than the trunk and limbs.
All components of normal skin have a role to play in relation to the barrier function, not only the epidermis and dermis with their vigilant reactivity to many stimuli and their innate immune function, but also the subcutaneous tissues (fat and connective tissue), the many cells and the interstitial matrix. The immunological functions of the skin depend both on cells in the epidermis and on dermal cells. An adequate blood supply and lymphatic drainage and a (mainly sensitive) innervation guarantee homeostasis of all involved components, including the pilosebaceous units or sweat glands.
Vulnerable skin may be malfunctioning in one or more of the aspects described above, by giving way to mechanical stress and tearing, by not being able to balance biological homeostasis or by defective immunological functions. See Figure 3.
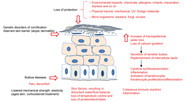
'Skin failure' (see Figure 4) has been defined by Irvine (1991) as a loss of normal temperature control or a failure to prevent percutaneous loss of fluid, electrolytes and protein, or as a failure of the mechanical barrier to prevent penetration by foreign material and micro-organisms [7]. Ryan (1991) compared severe loss of skin function with the failure of other vital organ systems such as the heart, kidneys, liver or respiratory system, necessitating specialist treatment [8]. Vulnerable skin can also be caused by one or more of the following factors.
Skin thickness varies according to age, gender, anatomical site and even diurnal phases, owing to changes in dermal hydration caused by postural changes. The thickness of the respective layers will determine the mechanical properties of the epidermis, the dermis and subcutaneous fat. Skin is elastic to a degree and can be stretched for a few seconds by 10 to 50%. The tonus of the skin is maintained by the elastic fibres that restore extended skin. Continued extension beyond maximum tolerance causes irreversible stretching and changes in the collagen fibrils.
Skin ageing is accelerated by exposure to ultraviolet light. Old skin loses elasticity, its epidermal hydration is less well restored and turnover of cells and tissues may be slower. The skin of old people may thus become extremely thin and vulnerable (see Figures 5 and 6). Thinning of the epidermis is caused by a reduction in the number of cell layers; sometimes atrophy is very pronounced and scars or pseudo scars herald tears in the skin layers. The thickness of the horny layer frequently remains unchanged. Longstanding application or ingestion of corticosteroids may enhance this atrophy. Fat cells in the subcutis slow down their metabolic activity in old age, leading to a loss of fatty tissue.
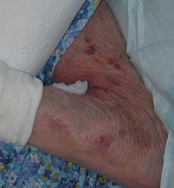
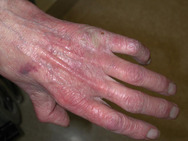
Skin dryness in old age is not so much due to the loss of the ability to retain water, but to a reduction in sebum production, secondary to low androgen titers. There is a complex interplay between the effects of oestrogens and androgens on many organs, including skin, in both men and women; sex steroids modulate many aspects of skin physiology, such as epidermal and dermal thickness, and they also influence immune system functions. Changes in hormonal levels with ageing alter processes such as skin surface pH, wound healing or propensity to develop autoimmune disease. Low oestrogen levels are thought to be responsible for changes in the hyaluronic acid content of the dermis, resulting in the low water-binding capacity of aged dermis.
If perfusion rates are lower, the skin may be pale, and the production of sebum, sweat and barrier substances is reduced. There may also be impaired thermoregulation and sometimes slower wound healing (see Figure 7). Loss of elasticity explains the passive distention by fluid during the day, resulting in oedema of varied tissular distribution according to several underlying causes and co-morbidities, typically in the legs and ankles (see Figure 8). In old skin the characteristic pattern of rete ridges (anchoring the epidermis to the underlying dermal tissue) flattens out, weakening the bond between the two layers of skin. Skin creases develop and pigment changes may be seen.
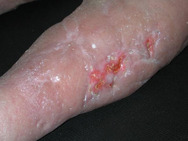
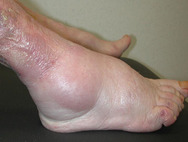
After injury the keratinocytes become activated and secrete various cytokines and growth factors to which they also respond. The type of injury will to a certain extent define the way in which keratinocytes elicit an appropriate reaction. On over-exposure to ultraviolet (UV) radiation, differentiation of keratinocytes is stimulated, and the enhanced cornification helps to provide protection against the damaging rays.
The skin has two barriers to UV radiation: a melanin barrier in the epidermis, and a protein-lipid barrier concentrated in the stratum corneum. In response to chronic sun exposure, thickening of the epidermis occurs. Shedding of damaged cells through desquamation is a way of preventing UV-induced carcinogenesis in the differentiating keratinocytes. In photodamaged skin the main contributing factors are probably alterations in the skin fibre structure of collagen and elastin, and accumulation of glycosaminoglycans with increased water-binding capacity in the subepidermal region.
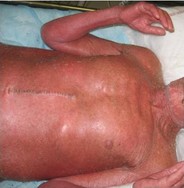
Pronounced atrophy of all layers of skin and subcutaneous tissues may be seen as a consequence of radiation damage. A precarious balance may exist for many years but then a minor trauma may precipitate a chronic wound with greatly impaired healing capacity. Connective tissue that has been damaged by radiation is similar to scar tissue, with an extreme paucity of cells, fewer feeding capillaries and atrophy of all irradiated skin layers. Figures 9 and 10 show skin damage caused by radiation therapy.
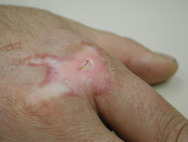
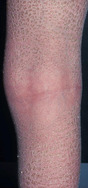
Genetic defects in lipid metabolism or in the protein components of the stratum corneum are also accompanied by skin barrier defects, such as in ichthyosis and related cornification disorders. Atopic dry skin has an impaired barrier function due to abnormalities in enzymes and lipids in the stratum corneum resulting in increased transepidermal water loss, lower water-binding capacity and vulnerability to Staphylococcus aureus colonisation. Examples are provided in Figures 11 and 12.
Skin vulnerability may be caused by genetic diseases such as bullous epidermolysis, which presents with several variations of anchoring defects between epidermis and dermis. In such patients, extreme vulnerability and susceptibility to mechanical trauma may render dressing changes very challenging.
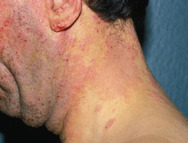
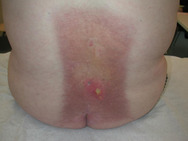
With advancing age and especially in patients suffering from long-standing diabetes, end-products of advanced glycoxidation and lipoxidation of structural molecules accumulate in the extracellular matrix of the skin (and other tissues). These change the biological behaviour of involved cells. If elimination of these metabolites is slowed because of impaired renal function, visible skin changes occur. Binding of water resulting in sub-epidermal oedema results in mechanical vulnerability: thin-roofed accumulations of local oedema will easily rupture under friction or pressure in that area. Leakage of fluid and lymph from the resulting erosions is unpleasant and necessitates frequent dressing changes, as well as permitting invasion by micro-organisms and allergens.
Inflammation of the skin is characterised by the migration of neutrophils, macrophages and lymphocytes, followed by a proliferation of endothelial cells and fibroblasts.
In inflammatory skin diseases infiltration of the skin is by inflammatory cells from the surrounding tissues or via diapedesis from blood vessels. Secreted proteins influence different enzymatic functions at the cellular and extracellular levels. Proteolysis of cell surface and extracellular matrix molecules is intrinsically linked to cell function. The inflammatory reaction can impact heavily on the epidermis and underlying dermis, profoundly disturbing their cellular turnover, maturation and function, and the synthesis of the skin-barrier elements. Both acute and chronic eczema result in vesicular lesions (see Figure 13), leading to erosions that become easily colonised or even infected. In desquamative inflammatory dermatoses, such as seborrheic dermatitis, psoriasis and eczema, skin barrier function is less effective.
Expression of ß-defensin-2 is upregulated by the inflammatory process in human skin. ß-defensin-2 also has a chemotactic and, in vivo, an activating effect on dendritic cells. In vivo recruitment of epidermal dendritic cell precursor from blood into skin and Langerhans cell mutation can also be influenced by other keratinocyte-derived factors. Chronic inflammation may lead to induration of skin and the subcutis, such as in venous insufficiency with lipodermatosclerosis, where soluble and membrane-bound metalloproteinases may favour enhanced turnover of the extracellular matrix in the lesional skin. In other circumstances, inflammation may lead to atrophy of skin and underlying tissues, as in scleroderma. Apoptosis of dermal and epidermal cells may result in ulceration.
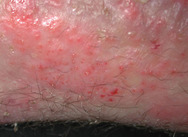
Accumulation of debris, sebum, remnants of topical formulations and of dressing materials will also induce inflammation (see Figure 14). Sweating and retention of water in wet and/or occluded areas or skin folds is the main cause of maceration and the development of intertriginous erosive skin lesions (Figure 15). The warm humid microclimate in these macerated areas facilitates colonisation or infection by fungi, yeasts and bacteria, enhancing the inflammatory reaction and leading to more swelling and oozing. This destroys the local barrier function of the skin. Infection, irritant dermatitis and sensitisation to topical substances will perpetuate a vicious cycle.
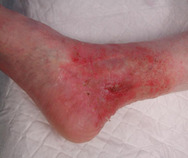
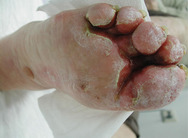
Repeatedly cleaning or rinsing the skin and wound using detergent or irritant substances may induce minor epidermal damage (see Figure 16). Initially, the reparative reaction may not be noticeable, but after a while the induced inflammatory repair is clinically visible as erythema (redness), scaling or desquamation, slight local swelling and sometimes vesiculation, fissuration and papules. Eczema or dermatitis may result from continued exposure to the responsible substance.
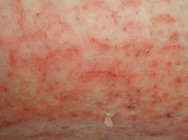
Immersion of the skin in water for an extended period of time or the use of solvents such as alcohol or ether extract lipid substances from the intercellular substance and dissolve the natural moisturising factor, affecting the water-retaining capacity of the skin barrier.
Normal human skin resists penetration by micro-organisms that routinely colonise its surface. Skin epidermal antimicrobial peptides and Langerhans cells are the most prominent factors in the defensive response. Two major classes of dermal peptides, cathelicidins and ß-defensins expressing antibacterial activity, are produced by keratinocytes. Moist lesions where the epidermal barrier is disrupted by a dermatological disease – such as atopic dermatitis – are readily colonised by S aureus. Adherence to epithelia and numbers of organisms correlate with the severity of the dermatitis [9].
Infection is a cause of acute and chronic inflammatory reactions, and patients with lymphoedema in particular may suffer from recurrent bouts of cellulitis. One of the best known complications in wound management is infection, often caused by resistant strains of bacteria, but also by facilitation of colonisation by micro-organisms such as yeasts and fungi or even virus particles. An erosive pustular dermatosis is generally ascribed to fungal infection of the skin under the moist and warm microenvironment induced by sustained multilayer bandaging.
In the presence of infection skin cells will activate the expression of tight-junction genes in order to prevent paracellular penetration of infectious viral particles [10].
After mechanical injury, keratinocytes release interleukin-1 (IL-1), which activates them and signal-alerts the surrounding tissues. The keratinocyte activation cycle is characterised by changes in expression of keratin proteins, enabling them to proliferate and migrate to repair the damage.
A similar situation is caused by stripping of the uppermost layer of the stratum corneum by forceful removal of adhesive dressings and tapes. This will inevitably result in a repair reaction as the skin attempts to compensate for the barrier by enhanced cell turnover in the underlying cell layers; this amplification is dependent on an inflammatory cascade of cellular events.
In current clinical practice, complications of wound management are the most frequent causes of prolonged morbidity and/or extension of hospital stay. Wound management complications may occur, even with the best possible care and precautionary measures.
Many factors and situations contribute to vulnerable periwound skin. Some are intrinsic, such as ageing, a genetic predisposition to certain skin conditions and skin changes caused by previous treatments. Some factors are extrinsic and could be prevented, such as the use of irritants, stripping by adhesives, occlusion and exposure to infection or allergens. Sore, irritated or broken skin can be a painful situation that impacts heavily on the patient's quality of life by increasing morbidity and prolonging a hospital stay.
1. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol 2008; 17(12): 1063-72.
2. Blanpain C, Fuchs E. p63: revving up epithelial stem-cell potential. Nat Cell Biol 2007; 9(7): 731-3.
3. Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays 2002; 24(9): 789-800.
4. Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol 2006; 19(6): 296-302.
5. Korting HC, Kober M, Mueller M, Braun-Falco O. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. Results of a cross-over trial in health probationers. Acta Derm Venereol 1987; 67(1): 41-7.
6. Yosipovitch G, Tur E, Cohen O, Rusecki Y. Skin surface pH in intertriginous areas in NIDDM patients. Possible correlation to candidal intertrigo. Diabetes Care 1993; 16(4): 560-3.
7. Irvine C. ‘Skin failure’ - a real entity: discussion paper. J R Soc Med 1991; 84(7): 412-3.
8. Ryan TJ. Disability in dermatology. Br J Hosp Med 1991; 46(1): 33-6.
9. Williams REA, Gibson A, Lever R, Aitcheson TC, MacKie RM. A comparison of quantitative sampling techniques of bacterial flora in atopic dermatitis and correlation with clinical state. Br J Dermatol 1989; 121(34): 39-42.
10. Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell 2005; 97(3): 173-83.