

|
Author(s)
Stephen Thomas
|
Contents
|
|
Published:
Mar 2008
Last updated: Mar 2008 Revision: 1.0 |
Keywords: maceration; moisture-related skin changes; exudate; ideal wound dressing; periwound skin.
If the moisture content of the skin varies significantly from normal values it can result either in dryness and cracking, or excoriation and the development of infection.
The term maceration is commonly used to describe changes to the skin resulting from prolonged exposure to water or moisture from sweat, urine or faeces. However, the widespread use of the term maceration can be misleading and may potentially lead to inappropriate treatments.
An alternative umbrella term is therefore proposed - 'moisture-related skin changes'. This encompasses skin changes such as superficial damage including nappy rash and intertrigo, as well as severe excoriation caused by the efflux of proteolytic enzymes from chronic wounds such as leg ulcers.
Unlike 'maceration' the proposed new term can also apply to adverse changes caused by insufficient moisture within a wound or areas of vulnerable tissue.
Local treatment of moisture-related skin damage generally involves the use of dressings with significant fluid handling properties to remove excess fluid from the wound and to provide protection to periwound skin. Sometimes, however, dressings are applied to donate or conserve moisture in order to prevent desiccation and tissue death.
This article describes the importance of controlling the moisture content of wounds and areas of vulnerable tissue, with particular emphasis on the use of dressings that provide protection to periwound skin, which may be damaged by proteolytic enzymes present in exudate from chronic wounds. It also discusses the importance of preventing excessive moisture loss from certain wound types and describes the way in which dressings can donate or conserve moisture in such situations.
The article also proposes a new definition for the 'ideal dressing' in the light of recent developments in the wound management field, which takes account of the requirement to protect vulnerable tissue from secondary damage caused either by insufficient or excessive moisture.
It is suggested that the correct application and frequent replacement of appropriate dressings, combined with the use of skin protectants or barrier creams where appropriate, will help prevent periwound damage, reduce the risk of infection and improve patient quality of life.
According to Wikipedia (http://en.wikipedia.org/) the term 'maceration' is derived from the Latin maceratus, the past participle of macerare, variously translated as 'to make wet, soak, steep or soften'. The free dictionary on line at http://www.thefreedictionary.com/ defines 'maceration' as the process by which an object or material 'becomes soft or is divided into its constituents by soaking'. The Online Medical Dictionary (http://cancerweb.ncl.ac.uk/omd/) goes further and defines maceration as a softening of tissue by soaking, especially in acids, until the connective tissue fibres are so dissolved that the tissue components can be teased apart.
In a clinical context, 'maceration' is commonly used to describe changes in the appearance of the skin, resulting from prolonged exposure to moisture or wound exudate, the causes and treatment of which have been described comprehensively in a series of articles [1] [2] [3] [4] [5] [6].
In the present article it is argued that the widespread use of the term 'maceration' can be misleading, and that it is sometimes applied incorrectly, potentially leading to inappropriate treatment in some instances. An alternative umbrella term, 'moisture-related skin changes', is therefore proposed. This encompasses maceration, but also includes other types of superficial skin damage such as intertrigo, and the irritation or excoriation caused by irritant body fluids such as urine, wound fluid or faecal material and/or the presence of pathogenic micro-organisms, their toxins and metabolites and inflammatory cytokines [6].
The skin, the largest organ in the human body with an area of approximately 1.8m▓, plays an important role in fluid regulation. The total volume of fluid held in the skin of a 70kg man is about 7 litres, but the moisture content varies throughout its structure. The dermis contains about 80% water and the stratum corneum about 30%, which is non-uniformly distributed, varying from around 40% in the inner layers to around 10-15% in the outermost horny layer [7]. This figure can increase to around 60% when the skin is immersed or exposed to a very wet environment [8].
Although the stratum corneum consists principally of layers of non-viable keratinised cells (corneocytes), the water content of this layer is crucial and is determined by at least three important mechanisms:
The transport of water from the dermis to the stratum corneum.
Moisture loss by evaporation (determined by intercellular lipids, which form a barrier to transepidermal water loss (TEWL)).
The water-binding ability of the stratum corneum itself.
This, in turn, is governed by the presence of intracellular water-soluble hygroscopic substances formed within the corneocytes by degradation of the histidine-rich protein known as filaggrin [9]. Together these comprise around 30% of the stratum corneum and are known as natural moisturising factor (NMF), consisting of 40% free amino acids, 12% prolidine carboxylic acid, 12% lactate and 7% urea, together with minerals, electrolytes and sugars. For a more comprehensive review of the fluid control mechanisms of the skin, see Agache and Black [10].
According to Verdier-SÚvrain and BontÚ [11], glycerol, a well-known cosmetic ingredient, has been discovered in the stratum corneum as a natural endogenous humectant. Hyaluronan, which is regarded principally as a dermal component, is also present in the epidermis, where it helps to maintain the structure and epidermal barrier function. A water-transporting protein, aquaporin-3, has additionally been discovered in the viable epidermis. All these findings have brought new insights into the mechanisms of skin water distribution and barrier function.
The NMF can be readily released or extracted from the cells of the stratum corneum with water after first treating it with solvents or detergents to extract protective polar lipids such as sphingolipids, which exist in the intercellular spaces [12]. It has also been demonstrated that repeated exposure to water can adversely affect the fluid control mechanisms of the skin by depletion of NMF even without prior solvent extraction [13].
If the fluid regulating ability of the skin is adversely affected, it makes it susceptible to dryness and scaling, particularly if the moisture content of the stratum corneum falls below about 10%.
Most people are familiar with the skin changes that occur after spending too much time immersed in a hot bath. These are characterised by pronounced softening, swelling and wrinkling of the epidermis, which certainly may be described as maceration according to the strict definition of the term. These effects are generally assumed to be caused by absorption of the bath water by the outer layer of the skin. The water then permeates the intercellular spaces, crosses cell membranes and swells the corneocytes [10].
Similar changes can also result from simple occlusion, for example by the extended use of rubber or plastic disposable gloves. The relatively impermeable nature of these materials prevents normal TEWL, which in turn leads to the accumulation of moisture within the skin and, ultimately, the same softening and wrinkling as described above.
In fact it is probable that the skin changes that occur from prolonged immersion in a bath result not just from absorption of water by the outer layers of the stratum corneum, but also by the accumulation of moisture in the deeper layers of the epidermis caused by the skin's inability to transpire excess water away in the form of sweat. In a hot bath, the situation is actually exacerbated by the fact that the capillaries within the skin are dilated as the body attempts to produce increased sweat as part of its normal temperature-regulating process.
These two simple examples clearly illustrate that major changes in the water content of the skin can be influenced by both endogenous and exogenous moisture.
Irrespective of the cause, in the two simple examples cited above, the obvious change in the thickness and appearance of the skin is reversible and therefore does not normally represent any serious threat to the individual concerned. However, while in this condition the skin is more susceptible to physical damage, and its protective barrier properties to chemicals and micro-organisms are impaired. Occluded (macerated) skin has also been shown experimentally to be more sensitive to irritants [14]. When exposed to a warm dry environment the skin returns to normal in minutes and no further treatment is indicated or required. (This desorption or water efflux is called skin surface water loss (SSWL), and is distinct from TEWL [10].) The application of any form of oily skin preparation or skin protectant at this stage would impair TEWL and thus delay the recovery process.
However, in the treatment of certain dermatological conditions associated with the formation of dry or cracked skin, such an effect may be clinically desirable. In such situations, oily emollients added to the bath, which form a film on the surface of the water, are transferred to the skin as the person rises out of the water. This thin oily layer helps to conserve any additional moisture taken up in the stratum corneum during bathing.
The skin changes described thus far are very different from those often observed around the margin of chronic wounds such as leg ulcers. While these skin changes may be partly due to maceration, which predisposes the affected area to traumatic injury (which may also be caused by some types of adhesive dressings), a second and potentially more important factor is the presence within chronic wound fluid of proteolytic enzymes. These can chemically degrade exposed skin, resulting in a red, weeping surface (see Figure 1).
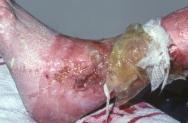
In such situations a barrier cream or a dressing that provides an effective seal to the periwound skin is probably indicated to provide a protective function. In one clinical study it was reported that maceration occurred in 55% of ulcers under investigation [15]. Maceration is a particular problem in diabetic ulcers [16], which in common with heavily exuding ulcers of all types, require frequent re-dressing to avoid or reduce damage to the surrounding skin [17] [18] (see Figure 2).
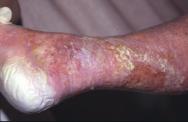
A third commonly encountered cause of superficial skin damage is the presence of urine or faeces on the skin surface, the irritant nature of which can also lead to superficial damage (see Figure 3). Diaper dermatitis is a common condition of which there are several types. When the occlusive effects of a nappy are not matched by its absorbency, hyperhydration of the stratum corneum occurs that progresses to maceration, increasing the coefficient of friction of the skin and predisposing to epidermal damage caused by rubbing. Faecal enzymes (urease, proteases and lipases) also can have a deleterious effect on the skin [19]. In elderly or immobile patients, maceration secondary to incontinence of urine or faeces is sometimes regarded as a precursor to skin damage caused by pressure and shearing effects, leading to pressure ulcer formation or extension [20].
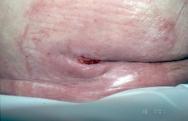
Obese or neglected children and adults are further subject to intertrigo - chafing or excoriation between moist skin folds or adjacent surfaces. All such areas are susceptible to infection with Candida albicans.
Where skin is at risk, it is possible to apply topical agents such as zinc paste or modern proprietary skin protectants [21] that are easier and quicker to apply and remove [22] and which have the additional advantage of being transparent.
Although the presence of liquid (be it urine, sweat or wound exudate) is undoubtedly a major contributory factor in all of these conditions, the skin does not have to be completely macerated in order for the damage to occur, which is why the term 'moisture-related skin changes' may be preferred.
In direct contrast, conditions that lead to a depletion of the moisture content of the skin can also produce visible changes of varying severity. Every year pharmaceutical and cosmetic companies spend millions of pounds in developing and promoting products designed to improve the moisture content of the skin and reduce the appearance of lines and wrinkles.
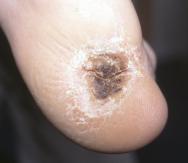
If the moisture content of the skin is seriously depleted, to below about 10%, it can result in dryness [23], leading to chapping or cracking, particularly on the fingertips or knuckles (see Figure 4). In extreme cases, total dehydration caused by death of the underlying dermal structures will lead to the formation of a dry black leathery eschar, commonly associated with pressure ulcers (see Figure 5).
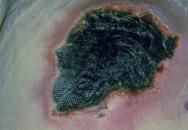
If the integrity of the epidermis is seriously compromised by trauma or some metabolic or physiological disorder, the healing rate of the resulting wound will be influenced by the moisture content of the surrounding skin and the local environment. Too dry and epithelialisation will be delayed, too wet and there is a risk of maceration and infection [24]. These conditions are determined principally by the choice of dressing [25].
In addition to facilitating healing, a product that maintains a moist environment can also help to prevent secondary damage to a vulnerable area of tissue that occurs as a result of dehydration. The capacity of deep partial thickness wounds to undergo spontaneous healing depends upon the survival of epidermal cells in hair follicles and sweat glands in the base of the wound; if these are allowed to become dehydrated and devitalised the wound may actually increase in size and convert from a partial-thickness to a full-thickness injury.
Work on burns reviewed by Lawrence [26] showed that the application of an occlusive dressing will salvage not only dermal tissue but also certain epithelial elements in the zone of stasis surrounding the original injury. The use of traditional dry dressings in these situations can result in progressive dehydration of the threatened zone followed by devitalisation and necrosis, with the result that this zone becomes indistinguishable from the original lesion. The prevention of dehydration by the application of a suitable occlusive or semipermeable dressing may limit or prevent these secondary effects.
In 1990 a number of key performance requirements of a dressing were identified, and a definition of the 'ideal dressing' proposed as follows [27]:
'The ideal dressing should provide an environment at the surface of the wound in which healing may take place at the maximum rate consistent with the production of a healed wound with an acceptable cosmetic appearance.'
In the intervening period, developments in the wound management field coupled with an increased understanding of the wound healing process, have been such that the list of functions that a dressing may be required to perform have been revisited.
These are summarised in Table 1, where they are divided into primary and secondary requirements. Primary requirements are those that are common to most wound management materials; secondary requirements relate to specific types of wounds or wounds in a particular condition or stage in the healing process. In both groups, the performance requirements have been divided into those which are determined principally by the design and construction of the dressing, over which the clinician has little or no influence, and those in which the ability of the product to perform in the required fashion is also influenced to a significant extent by the nature and condition of the wound.
| Primary requirements | Type of feature |
| Free of toxic or irritant extractables | Design feature |
| Does not release particles or non-biodegradable fibres into the wound | Design feature |
| Forms an effective bacterial barrier (effectively contains exudate or cellular debris to prevent the transmission of micro-organisms into or out of the wound) | Design feature |
| If self-adhesive, forms an effective water-resistant seal to the periwound skin, but is easily removable without causing trauma or skin stripping | Design feature |
| Maintains the wound and the surrounding skin in an optimum state of hydration (this implies the ability to function effectively under compression) | Design/wound related |
| Requires minimal disturbance or replacement | Design/wound related |
| Provides protection to the periwound skin from potentially irritant wound exudate and excess moisture | Design/wound related |
| Produces minimal pain during application or removal as a result of adherence to the wound surface | Design/wound related |
| Maintains the wound at the optimum temperature and pH | Design/wound related |
| Secondary requirements | Type of feature |
| Possesses antimicrobial activity - capable of combating localised infection | Design feature |
| Has odour absorbing/combating properties | Design feature |
| Has ability to remove or inactivate proteolytic enzymes in chronic wound fluid | Design feature |
| Possesses haemostatic activity | Design feature |
| Exhibits effective wound cleansing (debriding) activity | Design/wound related |
Even a cursory review of the performance requirements identified in Table 1 will indicate that it is unlikely that a single dressing or dressing system will possess all of these attributes.
A dressing that is ideally suited to the early stages of the treatment of infected, malodorous or necrotic wounds may not be appropriate for the later stages of healing. For example, sterile maggots represent arguably the most rapid and cost-effective non-surgical method for achieving a clean wound bed, but few would suggest that they should be applied to all types of wounds throughout the entire healing process. Similarly, a dressing that promotes angiogenesis and the production of granulation tissue may not be equally suitable for the final epithelialisation stage of wound closure. And a film dressing that may provide ideal conditions for the final stages in the healing process of an epithelialising wound, will not be suitable for a slough-filled heavily exuding infected leg ulcer.
A modified definition of the 'ideal dressing' is therefore offered, as shown below. As it is also the case that a combination of different components may be required to produce the required conditions for a given wound at a specific stage in the healing process, the definition has been modified to reflect this by making reference to a 'dressing system':
'The ideal dressing or dressing system provides an environment within the wound in which the objectives of the current phase of treatment may be achieved in a timely and cost-effective manner without compromising either the patient's safety or quality of life, or adversely affecting the integrity of the periwound skin or the final cosmetic appearance of the healed wound where this is relevant.'
It will be seen that this definition applies equally to products designed to achieve debridement, combat odour or infection, or promote granulation or epithelialisation. It follows, therefore, that for some wounds, but by no means all, optimal wound management may involve the sequential application of a number of 'ideal dressings' that are selected according to the condition of the wound as it progresses towards healing.
In the list of dressing functions identified previously, the primary performance requirements that are influenced by the condition of the wound are directly or indirectly related to the management of exudate or TEWL - the principal causes of maceration.
In clinical practice, although some dressings such as hydrogels are used to rehydrate eschar in order to promote autolytic debridement, the majority are applied to remove excess wound fluid (exudate) from the immediate vicinity of the wound.
With the exception of the vacuum assisted closure technique (VAC« Therapy), in which fluid is actively withdrawn from the vicinity of the wound before it has time to spread onto the surrounding skin, exudate control with many advanced and traditional types of dressings is commonly achieved by one or more different mechanisms. Most products possess some absorptive capacity that may be provided by means of absorbent fibres or foam that rapidly take up fluid from the wound surface. Depending on the design of the dressing, this is then distributed throughout the body of the absorbent layer, spreading both laterally and vertically towards the outer surface. At this point a second mechanism of action may come into play in which the absorbed fluid comes into contact with gel-forming agents located within or behind the absorbent layer. These gel formers may be derivatives of starch, or superabsorbent polymers that possess a remarkable affinity for liquid. Sometimes, as in the case of dressings made from alginate, the absorbent and gel-forming layers are one and the same.
The presence of materials with high absorption retention greatly improves the ability of a dressing to retain liquid under pressure as the fluid is 'locked away' within its structure. This can be particularly important in the case of products made from foam which, although capable of taking up significant volumes of fluid, do not necessarily retain this well under compression.
It is self-evident that the absorbent capacity of any dressing is finite, limited by size (area) and volume, and although in theory it is possible to increase the absorbent capacity by simply increasing dressing thickness, this impacts negatively upon conformability and patient comfort.
A third fluid handling mechanism is therefore often employed, which involves the incorporation of a semipermeable film or foam backing layer into the dressing's structure. Such layers permit the loss of fluid by evaporation through the back of the dressing while preventing the ingress or egress of liquid or micro-organisms. Unlike the finite absorption capacity, the ability of the dressing to cope with exudate by evaporation is relatively unlimited, and is determined only by the permeability of the membrane relative to the rate of exudate production by the wound. The relative importance of these fluid handling mechanisms is determined by the structure, composition and physical characteristics of the various components from which the dressing or dressing system is constructed.
A further important component included in many dressings is a wound contact layer that is designed to reduce the possibility of adherence of the dressing to the surface of a drying wound. Available in many forms, including perforated plastic films or nets, these layers are most commonly used in dressings that have an absorbent layer made from foam or cellulose fibre.
Many dressings, as they take up fluid, transport this laterally throughout the absorbent layer, including the inner surface that is in direct contact with the skin. This can increase the moisture content at the skin surface, which in turn may lead to maceration or other moisture-related effects described previously. The moist layer forms a pathway along which micro-organisms can migrate, either into or out of the wound.
The presence of a suitable wound contact layer can reduce although not eliminate this effect entirely as it forms an interface layer that physically separates the moist dressing surface from the periwound skin. If the wound is heavily exuding and the absorbent capacity of the dressing is insufficient to cope with all the fluids produced, exudate may accumulate in the defect and then gradually spread across the surface of the skin beneath the wound contact layer.
In the case of a chronic wound that contains high concentrations of proteolytic enzymes, this may lead to excoriation of the skin with consequent enlargement of the wound itself.
An alternative method of preventing this problem involves the use of a dressing that forms an adhesive bond or seal with the skin right up to the margin of the wound. An early example of such a product is the hydrocolloid dressing, which has one surface that is uniformly coated with an adhesive gel-forming mass. Provided that the seal remains intact, such a dressing is able to form a very effective protective covering to the healthy skin while absorbing (gelling) exudate in the immediate vicinity of the wound. If the adhesive seal fails around the wound margin, however, exudate contained within the vicinity of the wound will escape and flood over the skin, leading to maceration or excoriation by the mechanisms described previously.
A similar sealing effect is achieved in products coated with soft silicone technology in which an absorbent layer of foam, backed with a polyurethane membrane, is coated with a layer of soft silicone that forms a gentle bond or seal between the dressing and the wound in order to ensure that fluid is taken up by the dressing and does not escape on to the surface of the skin. In clinical studies such dressings have been shown to facilitate vertical wicking and reduce periwound maceration and pain [28] [29].
Such products differ from dressings in which an absorbent pad is located in the centre of a sheet of foam or film coated with adhesive to form an island dressing. When selecting such a dressing its island area must be larger than the wound itself so that it covers the wound area by some distance and overlaps the surrounding skin. In such situations the possibility of lateral transfer of exudate cannot be excluded.
A soft silicone dressing was compared with an island dressing in a clinical study involving 38 patients. Although healing rates were similar in the two treatment groups, as might be predicted, the incidence of maceration and local skin damage was significantly greater in the group treated with the island dressing [28].
It is vital, however, that all dressings used in this way are either sufficiently permeable to moisture vapour or have sufficient fluid affinity to cope with TEWL through the intact skin. If the dressing is occlusive or the adhesive layer does not have the capability to absorb the required amounts of moisture, fluid will accumulate beneath the dressing, potentially leading to maceration of the skin and/or a failure of the adhesive bond.
Hard data on the effect of moisture-related skin changes on treatment costs are hard to find, and the author is unaware of any dressing studies specifically focused on this area. It is not unreasonable to assume, however, that secondary damage caused in this way will delay healing and extend treatment times - with obvious financial implications for dressing usage, nursing time and, potentially, extended periods of hospitalisation. It will also have a negative impact on the patient's quality of life. If the skin changes also contribute to the development of infection, there may be additional costs for systemic antimicrobial therapy. As in many areas of clinical practice, prevention of moisture-related skin changes is better (and cheaper) than cure.
Given the multiplicity of dressings available and the clinical and financial implications of significant maceration, it might be supposed that the medical literature would contain a wealth of information on the treatment of this condition. In fact, in 2007 a systematic review of the literature relating to the management of maceration of the periwound skin [6] identified nine relevant articles and in only six of these was maceration cited as a primary or secondary outcome variable.
The authors also considered the evidence for the use of honey, topical negative pressure therapy, compression therapy and the use of a skin protectant. While there was reasonably strong evidence to support the use of skin protectants, the authors found no supporting evidence for the other treatment modalities [6].
In the absence of hard evidence from controlled studies, best practice standards based on expert opinion supported by laboratory or other experimental data (where relevant) must be used to guide clinical practice. It must also be remembered that absence of evidence of effectiveness is not the same as evidence of ineffectiveness.
For example, despite the lack of published data, many clinicians would accept that the use of compression, which reduces oedema and exudate production, will almost inevitably impact upon maceration. Similarly the application of topical negative pressure, which continuously removes exudate from the immediate vicinity of a wound, will reduce the possibility of skin damage caused by the spread of irritant wound fluid over the periwound skin. However, when using this technique, it is important to consider the moisture vapour permeability of the film component of the dressing system for if this is too low it could actually cause maceration of the periwound skin by preventing TEWL.
In most instances it is likely that periwound skin damage, regardless of its primary cause, may be prevented by the adoption of simple measures including the application and frequent replacement of appropriate dressings, and the use of skin protectants or barrier creams combined with good nursing practice. These simple measures should impact favourably on the patient's quality of life by reducing the pain and some of the inconvenience associated with a heavily exuding wound.
Historically, the selection of a dressing was determined by a number of factors, the majority of which were related to the position and nature of the wound, with particular attention paid to the presence of infection, odour and the amount of exudate present. Relatively little attention was given to the management of periwound skin or the effect that the choice of dressing system might have on this potentially vulnerable area. It was often tacitly accepted that wound fluid would inevitably escape from a heavily exuding leg ulcer on to the surrounding skin under the effects of gravity and there was relatively little that could be done to prevent this, other than to apply large quantities of bulky padding in an attempt to absorb the excess fluid. Products made from alginate or carboxymethylcellulose fibre are particularly prone to this problem, especially if they are not used with appropriate secondary dressings [30].
Modern dressings with enhanced fluid handling capabilities, such as new types of highly permeable foam/film combinations [31], foams containing superabsorbents, and highly absorbent hydrogel sheets, offer substantial advantages over products used in the past and have done much to alleviate this problem. The situation has been further improved by the advent of effective skin-friendly adhesive systems which form an effective seal between the dressing and the skin around the wound margin, and which also permit replacement of dressings without the production of the pain or trauma sometimes encountered with traditional adhesive materials [29].
1. Cutting KF. The causes and prevention of maceration of the skin. J Wound Care 1999; 8(4): 200-1.
2. Cutting KF. The causes and prevention of maceration of the skin. Prof Nurse 2001; 17(3): 177-8.
3. Cutting KF, White RJ. Avoidance and management of periwound maceration of the skin. Prof Nurse 2002; 18(1): 33, 35-36.
4. Cutting KF, White RJ. Maceration of the skin and wound bed. 1: Its nature and causes. J Wound Care 2002; 11(7): 275-8.
5. White RJ, Cutting KF. Interventions to avoid maceration of the skin and wound bed. Br J Nurs 2003; 12(20): 1186-201.
6. Gray M, Weir D. Prevention and treatment of moisture-associated skin damage (maceration) in the periwound skin. J Wound Ostomy Continence Nurs 2007; 34(2): 153-7.
7. Agache P. Stratum corneum histopathology. In: Agache P, Humbert P, editors. Measuring the Skin. Berlin: Springer-Verlag, 2004.
8. Bouwstra JA, Gooris GS, van der Spek JA, Bras W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J Invest Dermatol 1991; 97(6): 1005-12.
9. Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther 2004; 17(Suppl 1): 43-8.
10. Agache P, Black D. Stratum corneum dynamic hydration tests. In: Agache P, Humbert P, editors. Measuring the Skin. Berlin: Springer-Verlag, 2004.
11. Verdier-SÚvrain S, BontÚ F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol 2007; 6(2): 75-82.
12. Yamamura T, Tezuka T. The water-holding capacity of the stratum corneum measured by 1H-NMR. J Invest Dermatol 1989; 93(1): 160-4.
13. Visscher MO, Tolia GT, Wickett RR, Hoath SB. Effect of soaking and natural moisturizing factor on stratum corneum water-handling properties. J Cosmet Sci 2003; 54(3): 289-300.
14. Basketter D, Gilpin G, Kuhn M, Lawrence D, Reynolds F, Whittle E. Patch tests versus use tests in skin irritation risk assessment. Contact Dermatitis 1998; 39(5): 252-6.
15. J°rgensen B, Price P, Andersen KE, Gottrup F, Bech-Thomsen N, Scanlon E, et al. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J 2005; 2(1): 64-73.
16. Rodgers A, Watret L. Maceration and its effect on periwound margins. Diabetic Foot 2003; 6(3 Suppl): S2-S5.
17. Hilton JR, Williams DT, Beuker B, Miller DR, Harding KG. Wound dressings in diabetic foot disease. Clin Infect Dis 2004; 39(Suppl 2): S100-3.
18. European Wound Management Association (EWMA). Position Document. Wound Bed Preparation in Practice. London: MEP Ltd, 2004; available from URL: http://www.ewma.org/.
19. Flagothier C, PiÚrard-Franchimont C, PiÚrard GE. [How I explore ... diaper dermatitis] (article in French). Rev Med Liege 2004; 59(2): 106-9.
20. Doughty D, Ramundo J, Bonham P, Beitz J, Erwin-Toth P, Anderson R, et al. Issues and challenges in staging of pressure ulcers. J Wound Ostomy Continence Nurs 2006; 33(2): 125-30; quiz 131-2.
21. Campbell K, Woodbury MG, Whittle H, Labate T, Hoskin A. A clinical evaluation of 3M no sting barrier film. Ostomy Wound Manage 2000; 46(1): 24-30.
22. Cameron J, Hofman D, Wilson J, Cherry G. Comparison of two periwound skin protectants in venous leg ulcers: a randomised controlled trial. J Wound Care 2005; 14(5): 233-6.
23. Siddappa K. Dry skin conditions, eczema and emollients in their management. Indian J Dermatol Venereol Leprol 2003; 69(2): 69-75.
24. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962; 193: 293-4.
25. Winter GD. A note on wound healing under dressings with special reference to perforated-film dressings. J Invest Dermatol 1965; 45(4): 299-302.
26. Lawrence JC. Laboratory studies of dressings. In: Lawrence JC, editor. Wound Healing Symposium (Birmingham, 1982). Oxford: Medicine Publishing Foundation, 115-28.
27. Thomas S. Wound Management and Dressings. London: Pharmaceutical Press, 1990.
28. Maume S, Van De Looverbosch D, Heyman H, Romanelli M, Ciangherotti A, Charpin S. A study to compare a new self-adherent soft silicone dressing with a self-adherent polymer dressing in stage II pressure ulcers. Ostomy Wound Manage 2003; 49(9): 44-51.
29. Cunningham D. Treating venous insufficiency ulcers with soft silicone dressing. Ostomy Wound Manage 2005; 51(11A Suppl): 19-20.
30. Thomas S. The importance of secondary dressings in wound care. J Wound Care 1998; 7(4): 189-92.
31. Thomas S. Fluid handling properties of Allevyn foam dressing. BST Publications, 2007; available from URL: http://www.medetec.co.uk/Documents/Fluid%20handling%20properties%20of%20Allevyn%20foam%20dressing24-4-07.pdf.