

|
Author(s)
Marta Carlesimo,
Carmen Cantisani
Elena Mari
Mauro La Pietra
Alfredo Rossi
Vincent W Li
Stefano Calvieri
|
Contents
|
|
Published:
Jan 2007
Last updated: Jan 2007 Revision: 1.0 |
Keywords: pyoderma gangrenosum; intravenous immune globulin (IVIG); neutrophilic dermatosis; ulcerative process.
Pyoderma gangrenosum is a rapidly evolving and severely debilitating skin disease. Ulcers are often deep and painful, characterised by violaceous (intense purple-coloured) margins.
The aetiology of pyoderma gangrenosum is often unclear. It may be idiopathic or associated with a history of inflammatory bowel disease, including Crohn's disease, or haematological or rheumatic disorders.
Untreated pyoderma gangrenosum may last several months or years.
Treatment of pyoderma gangrenosum usually involves systemic therapy with high-dose steroids.
Intravenous immune globulin (IVIG) may be used where ulceration is resistant to conventional treatment.
Pyoderma gangrenosum is a neutrophilic dermatosis that may be idiopathic or associated with an underlying disease. It is the result of an exaggerated response to specific and non-specific stimuli. First-line treatment is with a steroid, usually prednisone. In this case report, a 25-year-old man presented with an ulcerated lesion that had appeared spontaneously one year previously. He was treated with prednisone, but this was ineffective. Treatment with intravenous immune globulin (IVIG), however, proved successful. IVIG contains a broad range of antibodies that act against bacterial and viral antigens and is used to treat inflammatory and autoimmune diseases. Its use is therefore recommended for consideration in cases of pyoderma gangrenosum that are steroid resistant.
First described in 1930, pyoderma gangrenosum is a non-infectious neutrophilic dermatosis [1]. The condition has an idiopathic form as well as one associated with an underlying disease such as inflammatory bowel disease, arthritis, haematological disease, human immunodeficiency syndromes and solid tumours [2] [3]. There is also an idiosyncratic form that can be triggered by certain drugs [4].
The classic skin lesion of pyoderma gangrenosum usually begins with folliculocentric pustules or fluctuant nodules with an inflammatory halo, and expands peripherally to form an ulcer with sharply circumscribed violaceous raised edges [1]. Lesions typically affect the lower extremities and the trunk [4]. The varied appearance of these ulcers has led to their recent clinical classification, which includes four prototypic forms of pyoderma gangrenosum: ulcerative, pustular, bullous and vegetative. Only the last of these classifications has no common association with underlying systemic disease. Each form may develop into another or become ulcerative [4] [5]. The diagnosis does not depend on histological biopsy findings and is especially challenging in its initial clinical form. A clinical-histological approach is required in order to make the diagnosis and to exclude other ulcerative processes involving dermal neutrophilia [4].
The first line of treatment for pyoderma gangrenosum is usually the use of systemic corticosteroids, together with treatment of any underlying cause [5]. Many therapeutic approaches have been reported with inconsistent results [5]. IVIG has been used in inflammatory and autoimmune diseases, but there are very few reports of its use in cases of pyoderma gangrenosum [6] [7] [8] [9]. The following case report describes a patient with pyoderma gangrenosum recalcitrant to conventional treatment, who was treated successfully with IVIG.
A 25-year-old Caucasian male was referred to our department for evaluation of an ulcerated lesion in the left malleolar region. This lesion had appeared spontaneously one year before as a red violaceous papule with an inflammatory halo that ulcerated after one month. The ulcerated lesion had sharply circumscribed, undermined and painful borders with evidence of residual bulla and pustules (Figure 1). Surgical skin grafting was performed, but this resulted in tissue pathergy and worsening of the ulcer. Pathergy refers to either a subnormal response to an allergen or, in the case of pyoderma gangrenosum, an unusually intense response, in which the individual becomes sensitive not only to the specific stimuli, but to others.
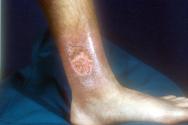
The clinical appearance was consistent with a diagnosis of pyoderma gangrenosum. The biopsy specimen demonstrated a dense diffuse neutrophilic infiltrate with a mixture of lymphocytes, plasma cells, histiocytes and occasional foreign body giant cells that extended to the subcutis.
All laboratory evaluations were within normal limits except for the lymphocyte subpopulation (Table 1). These findings show an alteration in cell-mediated immune response, with an uncontrolled response to non-specific stimuli.
| T-cell subsets: CD (cluster differentiation) | Results (percentage) | Normal value (percentage) |
| CD3 | 68.1 | 59-75 |
| CD4 | 21.8 (below normal value) | 40-53 |
| CD8 | 47.3 (above normal value) | 22-36 |
| CD56 | 26.5 (above normal value) | 1-7 |
| CD2 | 74.5 | 60-80 |
| CD19 | 3.2 (below normal value) | 4-12 |
| CD4/CD8 | 0.5 (below normal value) | 1.8+/-0.5 |
As pyoderma gangrenosum can be associated with HIV, an HIV test was performed and found to be negative. Other diagnostic tests such as chest X-ray, abdominal ultrasound and a double contrast barium enema, performed in order to exclude any underling diseases, revealed no abnormalities.
A conventional treatment regimen for pyoderma gangrenosum was tried initially, using oral prednisone tablets 40mg/kg/day for one month, scaling down the following month. This was discontinued because of a lack of improvement (Figure 2)
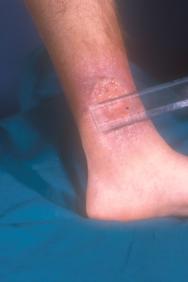
Oral cyclosporine tablets 2.5mg/kg/day were then introduced for one week. However, the patient developed hypertension and treatment was discontinued. A decision was taken to treat the patient with IVIG, as follows:
A dose of 27.5g was given on four consecutive days in month one
A dose of 55g was given on two consecutive days in month three
A dose of 55g was given over two consecutive days at intervals of three months, then five months and then two months.
A dramatic improvement was observed in the ulcer following the first dose, resulting in rapid healing without any significant side effects. Over a three-year follow-up period, there was no evidence of recurrence (see Figures 3 and 4).
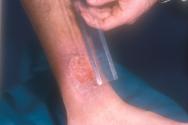
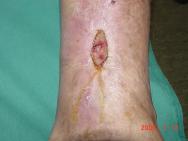
Since its first description in 1930, the aetiology of pyoderma gangrenosum has remained obscure [1]. Many hypotheses have been proposed, but attention has focused principally on immune abnormalities and alterations in cell-mediated immune response [10]. It is thought that pyoderma gangrenosum may be the result of a hyperergic (or hypersensitive) reaction of the immune system due to an altered, exaggerated and uncontrolled inflammatory response to specific and non-specific stimuli, leading to a neutrophilic vasculitis, characterised by perivascular deposition of immunoreactants, mainly immunoglobulin M (IgM), C3 and fibrin, with direct immunofluorescence. Neutrophils appear to play a key role in the pathogenesis of pyoderma gangrenosum. This is evidenced by the fact that the disease responds to therapies that have antineutrophilic activity. In some patients defective cell-mediated immunity has been identified, including: defective leukocyte adhesion glycoproteins; defective neutrophilic chemotaxis and intracellular killing of microbial pathogens; selective anergy (immune unresponsiveness) to bacterial or fungal antigens; and E hypergammaglobulinaemia.
When pyoderma gangrenosum is associated with systemic disease, the therapeutic approach should also address the underlying disorder [4] [11]. Treatment of lesions usually involves systemic treatment, together with local therapies [5] [11]. Systemic treatments include steroids such as prednisone 40-120mg/day until healing [8] [9] [10]. Although prednisone is a first-line treatment, it is not consistent in treating the condition successfully and the high doses required have been associated with significant side effects. Cyclosporine (6mg/kg) is a common therapy used either alone or in combination with steroids [12] [13]. Systemic antibiotics have also been used, including rifampin, tetracycline, vancomycin and mezocillin [8] [9]. Thalidomide has also been shown to be effective, especially in genital pyoderma gangrenosum [14].
IVIG is increasingly being used for the treatment of a range of inflammatory and autoimmune diseases [14]. IVIG is a sterile solution of immunoglobulins prepared from pooled human plasma. It contains a broad range of antibodies including IgG, and trace IgA and IgM, which act against bacterial and viral antigens. The mode of action is not well understood, but it appears to have numerous immune effects [5] [12] [13] [15]. These include:
Blocking of the Fc receptor expressed on the splenic macrophages with clearance reduction of Ic
Complement inhibition for the band between IVIG and C3b, C4b, without formation of lytic complex C5b-9
Inhibition of production of interleukin (IL)-1, IL2, IL3, IL4, IL5, IL10, tumour necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon-gamma (IFN-γ)
Neutralisation of auto-antibodies
Neutralisation of super-antigens
Block of Fas-receptor and apoptosis reduction of target cells
Apoptosis induction of auto-reactive T and B cells.
IVIG, acting on various parts of the immune system, can work as a 'reset' mechanism for different inflammatory and autoimmune diseases. The same mechanism may explain its success with pyoderma gangrenosum recalcitrant to conventional treatment. In the USA, IVIG is indicated for the treatment of immunodeficiency syndromes (for example agammaglobulinaemia, hypogammaglobulinaemia and Wiskott-Aldrich syndrome). Based on its putative mechanism of action and its tolerability, the use of IVIG could be expanded to treat a broad range of diseases.
IVIG can be considered as a treatment modality for pyoderma gangrenosum when conventional treatment has failed. In Europe, IVIG is a practical option (at a cost of approximately €284/55g in Italy). In the USA it is more expensive, at approximately $2500/55g. However, the cost of IVIG is reasonable when compared to the cost of many biologic agents, such as etanercept and infliximab.
In conclusion, IVIG should be considered as a well-tolerated and cost-effective treatment for steroid-resistant pyoderma gangrenosum.
1. Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11): 790-800.
2. Powell FC, Schroeter AL, Su WP, Perry HO. Pyoderma gangrenosum: a review of 86 patients. Q J Med 1985; 55(217): 173-86.
3. Rafael MR, Fernandes CM, Machado JM, Rodrigues PA, Cardoso OJ, Afonso A, et al. Pyoderma gangrenosum or leukaemia cutis? J Eur Acad Dermatol Venereol 2003; 17(4): 449-51.
4. Crowson AN, Mihm MC, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol 2003; 30(2): 97-107.
5. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005; 53(2): 273-83.
6. Bloom D, Fisher D, Dannenberg M. Pyoderma gangrenosum associated with hypogammaglobulinemia; report of two cases. AMA Arch Derm 1958; 77(4): 412-21.
7. Marcussen PV. Hypogammaglobulinemia in pyoderma gangrenosum. J Invest Dermatol 1955; 24(3): 275-80.
8. Barriere H, Litoux P, Stalder JF, Berger M, Delahaye M. Pyoderma gangrenosum associated with congenital hypogammaglobulinemia. Ann Dermatol Venereol 1979; 106(8-9): 695-6.
9. Caproni M, Pestelli E, Fabbri P. Le immunoglobuline endovenose in dermatologia. G Ital Dermatol Venereol 2003; 138: 377-83.
10. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: a review. MedGenMed 2001; 3(3): 6.
11. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol 1996; 34(3): 395-409; quiz 410-2.
12. Elgart G, Stover P, Larson K, Sutter C, Scheibner S, Davis B, et al. Treatment of pyoderma gangrenosum with cyclosporine: results in seven patients. J Am Acad Dermatol 1991; 24(1): 83-6.
13. Fradin MS, Ellis CN, Voorhees JJ. Management of patients and side effects during cyclosporine therapy for cutaneous disorders. J Am Acad Dermatol 1990; 23(6 Pt 2): 1265-73; discussion 1273-5.
14. Faver IR, Guerra SG, Su WP, el-Azhary R. Thalidomide for dermatology: a review of clinical uses and adverse effects. Int J Dermatol 2005; 44(1): 61-7.
15. Rutter A, Luger TA. High-dose intravenous immunoglobulins: an approach to treat severe immune-mediated and autoimmune diseases of the skin. J Am Acad Dermatol 2001; 44(6): 1010-24.