

|
Author(s)
Glen Cousquer
|
Contents
|
|
Published:
Feb 2006
Last updated: Feb 2006 Revision: 1.0 |
Keywords: Fly strike; myiasis; domestic rabbit; presentation; wound management; hydrogel dressings.
Domestic rabbits are vulnerable to fly strike, or 'myiasis', a condition most probably caused by the blowfly Lucilia sericata.
Rabbits with soiled hair and skin are particularly attractive to flies and such soiling can therefore predispose to fly strike. A wide range of factors may be responsible for such soiling and the underlying cause(s) should always be investigated.
An appropriate intensive care programme is required to stabilise the rabbit with fly strike. Fluid therapy, analgesia and antibiotic therapy are all indicated. Sedation or general anaesthesia is often necessary for wound cleaning and debridement. Any visible maggots should be removed. The wounds must be cleaned, debrided and dried; this process needs to be carried out repeatedly and thoroughly until all maggots are removed from the wound.
Use of hydrogel and hydrocolloid dressings will encourage wound healing.
Daily examination of all pet rabbits is necessary if fly strike is to be identified early. Attention to the rabbit's diet, activity levels and grooming, together with the use of a fly repellent, may help prevent recurrence.
Fly strike, or myiasis, is an extremely distressing condition affecting many rabbits during the summer months. Flies are attracted to lay their eggs on the rabbit under certain circumstances. The larvae, on hatching are able to attack healthy tissue and are capable of causing considerable soft tissue damage. This case report details the presentation, assessment and treatment of an obese two-year-old neutered male giant lop domestic rabbit (Oryctolagus cuniculus).
A two-year-old neutered male rabbit was seen for a routine vaccination and health check in February 2005. At the time he weighed 6.68kg and was receiving a high concentrate diet. A weight loss programme, consisting of a high fibre diet and increased exercise, was advised to get him back down to <6kg.
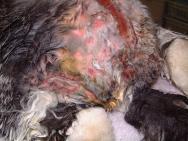
In October 2005, the rabbit presented as an emergency with severe fly strike. There was extensive skin ulceration over the dorsal midline, extending down on to the perineum. Significant numbers of first- and second-stage larvae were present. The rabbit was in a profound state of shock, showing weakness, depression, lethargy and anorexia. The rabbit weighed 6.76kg and had a rectal temperature of 38.8°C.
A shock therapy plan was instituted in order to stabilise the rabbit's condition. The rabbit was sedated with midazolam (Hypnovel) (2mg/kg intramuscularly). Anaesthesia was then induced by the administration of isoflurane by face mask. Intravenous fluid therapy was initiated via the right cephalic vein. A dose of 0.9% sodium chloride was initially infused as a continuous stream before reducing to 4ml/minute. Analgesia was provided with buprenorphine (Vetergesic) at 0.03mg/kg and carprofen (Rimadyl) at 4mg/kg, both subcutaneously. Antibiotic therapy consisted of enrofloxacin (Baytril) at 5mg/kg. All visible maggots were removed manually. In view of the severity of the injuries the owners were informed that the prognosis remained very guarded.
The rabbit was very subdued overnight but was accepting food and passing faeces by the morning. The rabbit remained subdued during the course of the day and was seen to pant a lot. Analgesia was continued with buprenorphine at 0.03mg/kg three times daily. The rabbit bit through its giving set during the morning of Day 2, forcing the suspension of intravenous fluid therapy. Maggot checks were performed three times daily, with the removal of a number of maggots on each occasion.
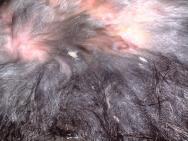
In the evening of Day 2 the rabbit was again sedated with midazolam (2mg/kg intravenously) in order to assess, further clean and debride the wounds (Figures 1 and 2).
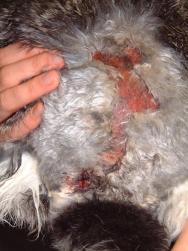

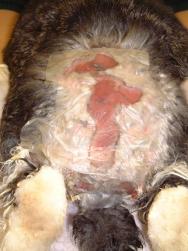
A catheter was placed in the marginal ear vein and 35ml saline, together with 5ml of a vitamin, mineral and amino acid supplement (Duphalyte), was administered intravenously. The hair over the rabbit's hindquarters was clipped using scissors. The wounds were gently scrubbed with a solution of dilute povidone iodine and subsequently lavaged liberally (Figures 3, 4, 5 and 6).
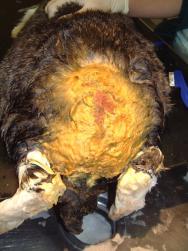

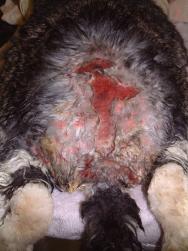
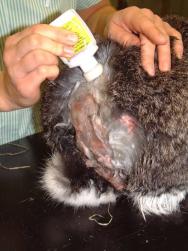
Following several cleaning cycles the rabbit was dried and its hair combed through to remove any remaining fly eggs (Figure 7). The wound was then dressed with a hydrogel (Intrasite) and covered with a moisture vapour-permeable dressing (Opsite) (Figure 8). An insect growth inhibitor (cyromazine - Rearguard) was applied to the skin and fur surrounding the wound (Figure 9). Analgesia was continued with buprenorphine three times daily, together with meloxicam (Metacam) at 0.3mg/kg once daily. A subcutaneous injection of ivermectin (0.2mg/kg) was also administered.
On Day 3, five maggot checks were performed and a single maggot removed. Analgesia with buprenorphine and meloxicam were continued. The ear catheter was well tolerated so bolus fluid therapy, consisting of 15ml saline plus 5ml of a vitamin, mineral and amino acid supplement (Duphalyte), was continued four times daily. Faecal and urinalysis were unremarkable.
On Day 4 a single maggot was recovered. The wound was flushed with dilute povidone iodine, rinsed and dried before reapplying a hydrogel/moisture vapour-permeable (Intrasite/Opsite) dressing. Buprenorphine was stopped and meloxicam used as the sole source of ongoing analgesia. The rabbit was drinking normally and appeared to resent the ear catheter, which was therefore removed.
Wound dressings were repeated daily for a further three days (until Day 7). During this time, antibiotic cover with enrofloxacin was maintained at 5mg/kg once daily.
On Day 7, the rabbit was anaesthetised for the purposes of applying a more resistant wound dressing. The rabbit was sedated with midazolam (2mg/kg) intravenously and then received ketamine at 15mg/kg. The rabbit was then intubated and maintained on isoflurane (1-3%) and oxygen. The wound was cleaned and debrided, before being dried. A sodium carboxymethylcellulose (Aquacel) dressing was applied to the exposed dermal tissues and a 10x10cm piece of thin hydrocolloid dressing (Duoderm Extra Thin) applied over the wound. This dressing was secured in place with simple interrupted sutures around the margin of the dressing (Figure 10).

The rabbit was discharged the following day (Day 8) with the dressing in place. Medication with meloxicam (0.3mg/kg once daily), together with enrofloxacin (5mg/kg once daily), was to be continued at home.
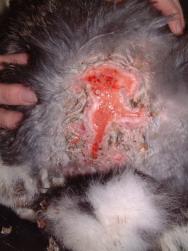
The rabbit re-presented on Days 11 and 15 for follow-up examinations. On Day 15, the remains of the dressing were removed. A healthy bed of granulation tissue was established and there was evidence of epithelialisation at the wound margins as well as at one or two islands (Figure 11). Figure 12 shows the healed rabbit.

Myiasis occurs when fly eggs are laid in damp areas of the skin or hair coat and the larvae develop in the underlying tissue of the host. In the US, larvae of Cuterebra species (or bot flies) may infect both wild and domestic rabbits, pupating under the surface of the skin [1]. In the UK, anecdotal evidence would suggest that the blowfly Lucilia sericata is largely responsible for incidences of myiasis in rabbits. Hall and Wall (1995) attribute myiasis of rabbits and other, usually debilitated, domestic and wild animals to L. sericata but no detailed studies have been published [2].
Flies such as L. sericata are attracted to soiled hair and skin. Activation, upwind orientation and landing appear to occur in response to putrefactive sulphur-rich volatiles originating from bacterial decomposition products. Oviposition is elicited primarily by the presence of ammonia-rich compounds, although moisture, pheromones and tactile stimuli are also reported to be attractive [3]. For various reasons rabbit skin and fur may become contaminated with faeces and/or urine. This is likely to prove attractive to flies and induce oviposition. An understanding of the factors likely to contribute to this situation is essential to both the management and prevention of fly strike in rabbits.
The various contributing factors that can attract flies to rabbits, and thus give rise to myiasis, are discussed by Cousquer (2006) [4]. These factors need to be considered and the underlying predisposing causes investigated. This is essential if the problem is to be prevented from recurring in the future. In the case of this rabbit, faecal and urinary examinations were unremarkable. This rabbit's main problem was its obesity. Obesity can make it difficult for a rabbit to groom itself and may make it difficult for it to demonstrate normal caecotrophy. Caecotrophs are usually eaten direct from the anus; this may not be possible in an obese rabbit and these soft motions may accumulate around the tail base as a result. Obese inactive rabbits may also soil themselves with urine. They may also contaminate their fur with faeces and urine from their hutch environment. Such contamination is likely to prove attractive to gravid female L. sericata flies, resulting in the deposition of eggs and subsequent myiasis.
Fly strike is always an emergency in any rabbit. The therapeutic plan must take into account a number of factors and it is suggested that the following be prioritised [4]:
Intravenous fluid therapy to combat shock
Analgesia
Antibiotic therapy
Removal of all second- and third-stage maggots
Removal of as many eggs and first-stage larvae as possible
Treatment to kill, or halt the development of, any remaining eggs and maggots
Wound management: debridement and dressing
Identification and treatment of any underlying causes
Nursing and supportive care considerations.
In the first instance, all patients require stabilisation. By the time fly strike has been noticed and the animal presented for veterinary care, the rabbit is usually in a state of profound shock. Intravenous fluid therapy is indicated at shock rates up to 90ml/kg/hour. During this time the rabbit should be carefully monitored for evidence of excessive fluid load. The lungs in particular should be auscultated regularly. Fluid rates should be reduced after 30-60 minutes to an appropriate rate, taking into account any outstanding fluid deficit and ongoing fluid losses. Use can be made of the cephalic or saphenous veins for this purpose. Where drip lines are chewed through, as occurred on Day 2 in this case, it may be appropriate to administer fluid by bolus injection. Ongoing fluid therapy is required until the rabbit is able to meet its own needs orally.
Analgesia must be provided to combat the severe pain associated with the enzymatic burns produced by the digestive enzymes of the larvae. Pain is often difficult to assess in rabbits and there is a risk that it may be underestimated. In this case the rabbit was hyperventilating for some time following admission. This is likely to have been a symptom of pain. Other symptoms included inactivity and anorexia in this case. It is safer to assume that a rabbit is experiencing pain and to provide good analgesia. A number of opioid analgesics can be used in rabbits. Buprenorphine provides six to eight hours of analgesia and is a good first choice [5]. Additional pain relief can be provided by the use of non-steroidal anti-inflammatory drugs such as carprofen and meloxicam. While neither drug is licensed for use in rabbits, both are widely used in rabbit medicine and appear to be well tolerated [5].
Where culture and sensitivity testing is not performed, use of broad-spectrum antibiotics is indicated. L. sericata is a facultative feeder and is likely to introduce a range of environmental bacterial contaminants. The possibility of secondary infection with Clostridial species must not be overlooked as this has been proposed as a possible cause of death in rabbits that appear stable post-surgery [1]. Secondary anaerobic bacterial infections can be treated with penicillin G procaine (30,000-60,000 IU/kg subcutaneously once every 24 hours for five days). Enrofloxacin and trimethoprim combinations are proposed by Harcourt Brown (2002) as safe choices [6]. In this case there was minimal necrotic tissue and the injuries did not extend deeper than the dermis. Antibiotic cover was therefore restricted to enrofloxacin.
Wound care and the removal of larger maggots are best performed under sedation or anaesthesia. The choice of sedatives will generally favour a combination that allows for a smooth induction and rapid recovery. In this case the rabbit received a sedative with anxiolytic properties. Anaesthesia was induced by the administration of isoflurane by face mask. This was well tolerated and allowed for a smooth induction. Where this is resented, a chamber induction can be less stressful. Isoflurane induction can be resented if performed with a face mask, especially in the unsedated rabbit, and is likely to induce breath holding [5]. Fentanyl/fluanisone provides sedation and effective analgesia and is a good alternative, favoured by some authors [6].
All second- and third-stage larvae should be identified and removed wherever possible. Second- and third-instar larvae are considered to be most damaging and their removal should be prioritised. These larger maggots are best removed manually using forceps and a systematic approach. The wound, as in this case, can initially be left undressed to allow repeat checks to be made for maggots.
Ideally, all eggs and first-stage larvae will be removed. This can be very intensive and requires repeat checks. Following wound lavage, many first-stage larvae are washed away. The coat then needs drying and, once dry, can be combed through to remove any remaining eggs that are left glued to the coat (Figure 7).
Any remaining eggs and maggots are likely to develop further. Use of an insect growth regulator can prevent the first larval instar metamorphosing into the second larval instar. Cyromazine (Rearguard) is licensed in the UK to prevent myiasis in rabbits. It should not be used on broken skin but can be applied to the surrounding fur and skin. In this case it was used to prevent the development of eggs and maggots that had not been removed manually. A number of other products have been proposed for use in rabbits suffering with myiasis. These include ivermectin and selamectin [1] [6] and permethrin. For a more detailed discussion of the available insecticides see Cousquer (2006) [4]. While topical treatment to kill blowfly larvae is desirable, use of a topical product in the presence of open wounds is likely to pose a greater risk of toxicity and so care is indicated in such circumstances [4].
L. sericata larvae produce a combination of proteinases with chymotrypsin-like and trypsin-like activities [7]. In the rabbit epidermal and dermal tissue appears vulnerable to the activity of these enzymes. It has been suggested that sheep and rabbits are unable to inactivate the proteolytic enzymes secreted by the larvae of L. sericata [8], and that, unlike humans, they are therefore vulnerable to myiasis as a primary condition. Vaccination of sheep with larval antigens has been shown to generate some antilarval protection. This effect was shown to be mediated by ingested ovine antibodies directed at proteins within the larval digestive tract [9]. No work has currently been conducted to determine what differences might exist between rabbits and humans that might explain differences in the invasiveness of L. sericata larvae. It is clear that both rabbits and sheep are capable of soiling their coats in such a way as to attract blowflies and induce them to lay eggs. This factor contributes to the susceptibility of rabbits to fly strike. It remains unclear, however, whether other factors make rabbits and sheep vulnerable to myiasis.
Practically, it appears that L. sericata larvae are able to penetrate through the epidermal and dermal layers of rabbit skin. The resultant enzymatic burns can be very extensive and require the development of an appropriate wound management plan [4]. In the first instance the wound must be cleaned of all organic material and debrided of all necrotic tissue. The removal of faecal and urinary contamination is required to eliminate the attraction to flies, while repeated wound lavage is required to facilitate the removal of bacteria, maggots and other organisms. In this case dilute povidone iodine, as proposed by Harcourt Brown (2002) was used to wash and disinfect the wounds [6]. The surrounding hair was clipped with scissors to minimise trauma to the skin. Even with scissors, however, great care is required to avoid nicking the skin. Once clean the coat and skin must be dried to avoid unnecessary chilling of the patient. Drying is also indicated to eliminate excess humidity as this may promote egg and larval development. The heat produced by a hairdryer will attract maggots towards it and can further facilitate their removal [6].
Once dry the wound requires protection. A wound dressing will protect the wound from ongoing fluid losses and minimise discomfort. The use of hydrogels such as Intrasite and Nu-Gel not only absorb bacteria and keep wounds hydrated but has also been shown to slow larval development [10]. It is therefore appropriate to apply hydrogels to fly strike wounds and cover the area with a moisture vapour-permeable dressing (such as Opsite). Repeat checks are required to ensure that any more maggots are identified and removed. In this case no further maggots were found after Day 4. Once it has been established that all maggots and eggs have been removed, a more permanent dressing can be applied. The objective is to provide protection to exposed nerve endings and provide a moist wound environment for the development of granulation tissue. Suitable dressing materials include hydrocolloid dressings (Granuflex and Duoderm Extra Thin), which can be sutured in place over a wound (Figure 10). The establishment of a healthy bed of granulation tissue will lead to a stage of rapid wound healing by secondary intention. This tissue must be protected as the rabbit hutch environment is particularly dusty. Use of non-dusty bedding during this period is desirable, provided the rabbit continues to receive plenty of long fibre in its diet.
Many rabbits succumb to myiasis because an underlying problem has not been identified and addressed. Faecal contamination and urine scalding are usually incriminated. It is essential that these problems be investigated and this is discussed further elsewhere [4]. In this case the patient was obese and relatively inactive. Such a large rabbit can experience difficulties keeping itself clean, especially if the environment is dirty. Obese rabbits are reportedly especially prone to myiasis [6]. The area at the base of the spine is not easily groomed and obese rabbits may experience particular difficulty in getting to this area; consequently this area is the commonest site for fly strike in rabbits [6].
Rabbits suffering from myiasis present clinicians and nursing staff with a number of challenges. Shock therapy and pain relief have already been discussed. The provision of a low stress environment is very important. Consideration must be given to what the rabbit is familiar with as house rabbits and garden rabbits may have different requirements. Generally speaking, rabbits should not be housed in the same room as dogs and cats, as these can be viewed as predators. A hiding place should always be provided to allow the rabbit to demonstrate escape behaviour when it is feeling threatened. A high standard of nursing care is required to ensure that the rabbit's recovery is facilitated. Attention must be paid to food intake and the provision of high fibre recovery formulas, such as Oxbow Critical Care, is to be encouraged. Small volumes of this can be syringe fed to the rabbit. It is, however, important that this is done patiently and calmly to minimise stress for the rabbit. In obese rabbits there is a significant risk of hepatic lipidosis developing if the rabbit remains inappetant. In such cases, analgesia, fluid therapy, syringe feeding and the use of motility stimulants are often necessary [6]. Food intake and faecal production must be closely monitored to ensure that the gastrointestinal tract is functioning normally.
Owners should remain alert to the risks presented by a rabbit with faecal or urine contamination. A twice-daily check should identify any such contamination and the underlying causes should be established in consultation with a veterinary surgeon. This point cannot be over-emphasised. Rabbits with perineal soiling should be carefully monitored and the cause of their soiling investigated without delay.
Once eggs have been laid, second-stage larvae can appear in as little as 38 hours. A more detailed discussion of the development of blowfly larvae is provided by Cousquer (2006) [4]. A single gravid female L. sericata is able to deposit about 200 eggs in a single egg batch and this can be enough to give rise to myiasis in a rabbit. This single fly can go on to produce as many as 21 generations [11], although in the field only three or four generations would be expected [12]. Increases in the fly population are directly proportional to environmental temperature, once the minimum threshold or 'base temperature', below which pre-adult stages do not develop and ovary maturation does not occur, has been exceeded. This base temperature was calculated to be around 9-11°C for L. sericata [12]. The season for flies therefore runs through the summer and can extend into October, as occurred in this case, if temperatures remain warm enough.
Biannual vaccination of rabbits allows a biannual health check to be performed. This should review all aspects of the husbandry and should include a dental examination and an assessment of the faeces. Clear guidance on the prevention of fly strike should be provided at this time.
The use of licensed preventative products is recommended in vulnerable individuals. The definition of vulnerable individuals includes all those prone to perineal soiling and should also include any rabbit being cared for by a carer who is unfamiliar with rabbits. This situation often arises when rabbits are left in the care of friends and family at holiday times [4]. Two products are marketed as insect repellents for use in rabbits within the UK. Xenex is a plant-based repellent containing octanoic and decanoic acid. Xenex Ultra Spot On contains permethrin and is recommended as a repellent as well as for its ability to kill adult flies and their larvae. Use of a fly screen may be beneficial, as may the use of a cattle fly tag (containing permethrin). Cattle fly tags should be nailed out of reach of the rabbit. The use of such products does not, however, obviate the need for addressing the underlying cause of perineal soiling.
Table 1 details the preventative actions and steps that can be taken in order to minimise the risk of fly strike in rabbits.
Diet Dental examinations Faecal checks Grooming Daily checks Fly repellents |
1. Hess L. Dermatologic diseases. In: Quesenberry KE, Carpenter JW, editors. Section 2 Rabbits. Ferrets, Rabbits and Rodents. Clinical Medicine and Surgery. Second edition. St Louis, MO: Saunders, 2004; 194-202.
2. Hall M, Wall R. Myiasis of humans and domestic animals. Adv Parasitol 1995; 35: 257-334.
3. Ashworth JR, Wall R. Responses of the sheep blowflies Lucilia sericata and L. cuprina to odour and the development of semiochemical baits. Med Vet Entomol 1994; 8(4): 303-9.
4. Cousquer GO. Veterinary care of rabbits with fly strike. In Practice 2006; In Press.
5. Flecknell PA. Anaesthesia. In: Flecknell PA, editor. BSAVA Manual of Rabbit Medicine and Surgery. Gloucester: BSAVA, 2000; 103-16.
6. Harcourt Brown F. Skin diseases. In: Textbook of Rabbit Medicine. London: Butterworth Heinemann, 2002; 224-48.
7. Chambers L, Woodrow S, Brown AP, Harris PD, Phillips D, Hall M, et al. Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol 2003; 148(1): 14-23.
8. Bell NJ, Thomas S. Use of sterile maggots to treat panniculitis in an aged donkey. Vet Rec 2001; 149(25): 768-70.
9. Tellam RL, Eisemann CH. Inhibition of growth of Lucilia cuprina larvae using serum from sheep vaccinated with first-instar larval antigens. Int J Parasitol 1998; 28(3): 439-50.
10. Thomas S, Andrews A. The effect of hydrogel dressings on maggot development. J Wound Care 1999; 8(2): 75-7.
11. Kamal AS. Comparative study of thirteen species of sarcosaprophagous Calliphridae and Sacrophagidae (Diptera). 1. Bionomics. Ann Entomological Soc Am 1958; 51: 261-71.
12. Wall R, French NP, Fenton A. Sheep blowfly strike: a model approach. Res Vet Sci 2000; 69(1): 1-9.