

|
Author(s)
Marco Romanelli
Madeleine Flanagan
|
Contents
|
|
Published:
Jul 2005
Last updated: Jul 2005 Revision: 1.0 |
Keywords: Pressure ulcers; wound bed preparation; chronic wounds; non-healing wounds; TIME framework; care of the elderly; advanced therapeutic products.
The treatment of pressure ulcers, particularly chronic or non-healing ulcers, places a significant burden on healthcare systems around the world. Pressure ulcers can also have a serious detrimental effect on quality of life, particularly among older people.
The concept of wound bed preparation and the TIME framework provide a rational and systematic approach to both the assessment and treatment of pressure ulcers.
A number of technological developments have greatly enhanced both local and systemic treatments for pressure ulcers.
Wound bed preparation is a relatively recent concept that can provide a structured approach to the management of chronic wounds [1]. Its strength is that it provides a rational and systematic approach to both assessment and treatment that is particularly useful when managing non-healing pressure ulcers. This article reviews wound bed preparation and the latest developments in the treatment of pressure ulcers in the context of the TIME framework (Tissue management, Inflammation and infection control, Moisture balance and Epithelial (edge) advancement) [1].
Pressure ulcers are a serious health issue: they cause great pain and suffering to patients and place a significant financial burden on healthcare systems. A recent European Pressure Ulcer Advisory Panel (EPUAP) study found that the overall prevalence of pressure ulcers in Europe was 18%, depending on patient group and care environment [2].
It is likely that with an ageing population and increasing longevity the number of patients with pressure ulcers or at risk of developing them will continue to rise in the future, placing greater strain on limited healthcare resources [3]. Preventive measures are essential to reduce prevalence rates, so healthcare professionals must be able to identify and adopt appropriate strategies to meet individual patient requirements.
The past decade has seen the development of a number of new therapeutic options for the management of pressure ulcers. The introduction of advanced medical devices and new approaches to systemic treatment have led to greater understanding of the mechanism of tissue repair in pressure ulcers, and standardised guidelines for prevention and treatment have been published. The aim of this paper is to apply the components of wound bed preparation and the TIME framework (Tissue management, Inflammation and infection control, Moisture balance and Epithelial (edge) advancement) [1] to the practical management of pressure ulcers. This framework will be used to describe the latest technological developments in local and systemic treatments for pressure ulcers. It is assumed that adequate pressure relief will also be provided in all settings.
Pressure ulcers have been defined as 'an area of localised damage to the skin and underlying tissue caused by pressure, shear, friction and/or a combination of these' [3]. Although the term 'pressure ulcer' is the most widely accepted because it accurately describes the nature of this type of lesion, alternative terms such as 'bedsores', 'decubitus' and 'pressure sores' have also been used.
The intensity and duration of pressure is a critical factor in the development of pressure ulcers, as is the skin's tolerance to pressure and its supporting structures. Pressure ulcers are traditionally graded according to the type and extent of tissue damage incurred. A variety of different grading models are available but there is some debate on which of these is the most accurate. For the purpose of this paper the EPUAP pressure ulcer classification system [3] will be used.
EPUAP pressure ulcer classification system:
Grade 1 - Non-blanchable erythema of intact skin. Discolouration of the skin, warmth, oedema, induration or hardness may also be used as indicators, particularly on individuals with darker skin.
Grade 2 - Partial-thickness skin loss involving epidermis, dermis, or both. The ulcer is superficial and presents clinically as an abrasion or blister.
Grade 3 - Full-thickness skin loss involving damage to or necrosis of subcutaneous tissue that may extend down to, but not through, underlying fascia.
Grade 4 - Extensive destruction, tissue necrosis or damage to muscle, bone or supporting structures, with or without full-thickness skin loss.
The first crucial step in the management of pressure ulcers is a holistic assessment of the patient, paying particular attention to any underlying pathologies. The effective management of patients with pressure ulcers or at risk of developing them is an interdisciplinary concern that must be reflected in a united approach that involves both patients and informal carers. Such an approach depends on organisational support, adequate resourcing and the implementation of best clinical practice, which should address all of the following issues:
the redistribution of pressure
nutritional assessment
continence management
pain control.
Nutrition plays a fundamental role in the prevention and treatment of pressure ulcers, so a well-balanced, nutritionally adequate diet should be a priority for all patients with pressure ulcers or at risk of developing them. Anaemia reduces the amount of oxygen available to the fibroblasts, inhibiting the synthesis of collagen and, therefore, the healing process. Low levels of serum albumin and hypoproteinaemia can result in oedema, which reduces cutaneous elasticity, as well as important changes in the microcirculation and a consequent delay in wound closure [4].
A series of European clinical guidelines have been formulated to define best practice in the prevention and treatment of pressure ulcers [3][5][6].
The removal of devitalised tissue from pressure ulcers is essential to prevent contamination, reduce tissue degradation and promote the development of healthy granulation tissue. Debridement also decreases bacterial burden and enhances visual assessment, which can improve accuracy when grading pressure ulcers.
Pressure ulcers with full-thickness skin loss (grades 3 and 4) are most commonly associated with extensive tissue damage and necrosis. Devitalised tissue can present as either thick, moist, offensive slough or dry, hard eschar. Several factors must be considered when deciding on the most appropriate method of debridement in patients with pressure ulcers. These include the grade, size and location of the wound; the patient's general condition; periwound moisture levels and pain management. Eschar in pressure ulcers need not be debrided if oedema, erythema, fluctuance or drainage are not present [3].
Two processes are commonly used to remove devitalised tissue and decrease the cellular burden of pressure ulcers: selective and non-selective debridement. Selective debridement is a more natural process than non-selective debridement because it uses the body's own enzymes, often in conjunction with exogenous enzymes, to remove only devitalised tissue. For this reason it is also usually less painful than non-selective debridement. In clinical practice a number of different debridement techniques may be used in the course of treating a large, necrotic pressure ulcer.
This includes the following techniques:
Autolytic debridement is a natural process that can be promoted through the use of hydrocolloid, hydrogel or calcium alginate dressings. These create a moist wound interface that enhances the activity of endogenous proteolytic enzymes within the wound, liquefying and separating necrotic tissue from healthy tissue [7]. This technique is particularly useful if, for technical or medical reasons, more invasive procedures are not suitable.
Enzymatic debridement is recommended for pressure ulcers covered by a dry, hard eschar, typically heel or sacral ulcers. Exogenous enzymatic preparations such as collagenase, which act in synergy with endogenous enzymes, can be applied to the wound bed [8] or eschar edge, encouraging it to separate from healthy tissue. Their penetration of necrotic tissue may be aided by crosshatching it with a scalpel, but this needs to be performed with care. In the case of cavity pressure ulcers with high exudate levels and wet, sloughy tissue, it may be best to consider an alternative method such as alginate dressings or biosurgical debridement (see below).
Biosurgical debridement uses sterile fly larvae to treat a range of wounds, including pressure ulcers. The sheep blowfly Lucilia sericata (Diptera: Calliphoridae) is the only maggot currently used for this purpose. Maggots secrete proteolytic enzymes that break down and liquefy tissue, which they then ingest. The enzymes produced by this species dissolve only dead tissue in human wounds and the maggots are therefore unable to damage healthy tissue. Maggot secretions also contain chemicals with inherent antimicrobial properties, which may help to combat infection by having an inhibitory effect on the growth of bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). Maggot therapy may result in more rapid debridement and less pain than some other therapies [9]. The main disadvantages are that some patients are averse to the use of maggots and that larvae may drown in deeper, heavily exuding pressure ulcers. In sacral pressure ulcers, friction forces may cause dressing slippage and leakage, making the use of larvae more problematic.
This includes the following techniques:
Mechanical debridment involves allowing a traditional gauze-type dressing to dry out and adhere to the surface of the wound before manually removing the dressing, debriding any tissue attached to it. This technique is not generally recommended as it is painful and may traumatise healthy or healing tissue. Hydrotherapy is another form of mechanical debridement, but waterborne pathogens may cause contamination or infection as well as tissue maceration.
Sharp or surgical debridement is the quickest way to remove the necrotic tissue and senescent cells that slow down the tissue repair process, converting a chronic wound into an acute one in the process. Certain conditions, such as advancing cellulitis or sepsis, require urgent surgical debridement, but the pain caused by the procedure and the risk of extensive bleeding must be considered during assessment. This technique requires skill and must always be performed by a competent practitioner, and EPUAP treatment guidelines recommend that sharp debridement should be carried out only if the patient's blood supply and general health can adequately support healing [3]. It should be noted that sharp debridement of larger chronic wounds may result in short-term bacteraemia, which may justify the administration of antibiotic prophylaxis.
Infected pressure ulcers can have many serious consequences, including heightened pain and anxiety, a deterioration in the patient's general condition and a detrimental effect on quality of life. The wound itself will increase in size, producing more exudate, slough and odour, and unresolved infection may ultimately lead to septicaemia and death.
Pressure ulcers are often colonised by multiple organisms, even though there may be no clinical sign of infection. These organisms originate mainly from skin flora or from the bowel as a result of faecal contamination, and typically include staphylococci, streptococci, Proteus mirabilis and Escherichia coli. Over the past decade the increasing incidence of MRSA and multi-resistant pseudomonas infections such as bacteraemia, pneumonia and surgical wound infections have made bacterial resistance a clinical priority in acute care facilities.
It is well documented that chronic wounds are important sources of MRSA colonisation [10][11] so pressure ulcers may act as reservoirs for multi-resistant bacterial strains. However, it can be difficult to determine the bacteriological status of a pressure ulcer because all exposed ulcers are colonised by bacteria. Cultures should be taken only if there are clinical signs of infection, but swab cultures detect surface contamination only and cannot reveal the micro-organisms responsible for tissue infection. Biopsy by aspiration is widely considered the gold standard technique to determine quantitative counts and identify bacteria, but this is often not practical in the clinical setting [12][13] [14].
If clinical infection is suspected the full range of relevant laboratory tests must be carried out and critically evaluated. In accordance with the clinician's experience and the evidence base from the literature, drug susceptibility tests should be considered.
There is an urgent need to rationalise the use of antibiotics when treating infected pressure ulcers as local ischaemia and the presence of devitalised tissue can prevent antibiotics from reaching the wound bed in sufficient doses to be therapeutic. Systemic antibiotics are not recommended for pressure ulcers that exhibit only clinical signs of local infection [3]. However, studies have shown a correlation between the incidence of deep-tissue infection and patients with multiple pathologies and poor general health. Systemic antibiotic use should be restricted to the treatment of cellulitis, osteomyelitis and bacteraemia [3]. These infections should be treated quickly using carefully selected antibiotics, with the correct dose prescribed for an appropriate period of time [15].
The final outcome for infected pressure ulcers depends on achieving a balance between factors that might create further complications and those that resolve existing problems. Host defence mechanisms are particularly important when assessing infected pressure ulcers as patients are often critically ill and have already received several courses of antibiotic therapy as part of their wound management programme. For these reasons all host defence mechanisms must be maximised, including maintaining adequate nutrition and correcting any electrolyte imbalance.
A number of different approaches can be used to reduce the bacterial burden in pressure ulcers to tolerable levels, including the use of antiseptics and systemic antibiotics in conjunction with maintenance debridement. Several types of topical antiseptics are used in wound management, but cadexomer-iodine, povidone-iodine and nanocrystalline silver have shown consistent efficacy in controlling bacterial burden in pressure ulcers and reducing the risk of resistance. These new slow-release antiseptics enable excellent control of resistant microbial species, such as MRSA and vancomycin-resistant enterococci, and can be used effectively as antimicrobial agents [16].
Cadexomer-iodine is an antimicrobial made up of a modified starch matrix to which iodine is physically bound. Wound exudate is absorbed by this matrix, while at the same time the iodine component of the dressing is slowly released into the wound in a controlled manner.
Povidone-iodine comprises iodine attached to an inert vinyl polymer matrix, which allows the iodine to be released into the wound in small amounts at a defined rate.
Nanocrystalline silver has a unique technology in terms of silver delivery that results in an effective biological action against a wide range of bacteria. Silver interacts with the cellular membranes of bacteria, causing structural changes that interrupt cell division and interfere with bacterial and electron transportation.
Superficial infection affects mainly grade 2 pressure ulcers and is characterised by the classic signs and symptoms of infection: delayed healing, a change in the colour of the wound bed, abnormal odour, increased exudate and pain, and friable granulation tissue [17]. In such cases, the application of topical antiseptics has been shown to be of great benefit in controlling bacterial burden and preventing systemic complications, and is an increasingly valuable alternative to the indiscriminate use of systemic antibiotics. However, antiseptics should not routinely be used to clean pressure ulcers and should be considered only when the bacterial load needs to be controlled, and then only for a limited period of time until the wound is clean and inflammation has been reduced [3].
Deep-tissue infection is a frequent complication of grade 3 and 4 pressure ulcers and is characterised by an increase in the temperature of the skin surrounding the wound, tenderness and pain. It is essential to determine the level of infection using a recognised pressure ulcer classification system, such as the EPUAP guidelines [3], to differentiate between superficial and deep-tissue infection because local infection could rapidly progress to systemic involvement. There may also be extended erythema, reaching to the bone and into new areas of breakdown.
Osteomyelitis is a common complication in infected pressure ulcers, with S. aureus the cause in about 60 percent of cases [18]. Any pressure ulcer with exposed bone is particularly at risk of osteomyelitis, which should be suspected if clinical signs of infection do not respond to conventional treatment. A diagnosis of osteomyelitis can be confirmed by blood culture and bone biopsy, and a prolonged parenteral therapeutic regimen lasting four to six weeks is often required.
Finally, the risk of infection and cross-contamination can be reduced by effective hand-washing and the use of clean gloves for each patient. Clinicians should remove their gloves and wash their hands after each patient contact. When treating multiple ulcers on the same patient, care should also be taken to attend to the most contaminated ulcer last [3].
Optimal moisture balance at the wound interface is a key element in wound healing. The level of wound moisture is related to several factors, including:
the type of wound
the phase of wound healing
the absorptive capacity of topical dressings.
The management of moisture is an essential aspect of wound bed preparation, but in pressure ulcers this can be particularly problematic due to increased levels of exudate and continence problems. Patients with pressure ulcers are often immobile and confined to bed, and ulcers are usually located on or near bony prominences, sites where the surrounding skin is vulnerable to the effects of moisture such as exudate, perspiration or urine. Skin moisture is one of the most frequently described factors in increased susceptibility to the external forces of pressure, shear and friction [3][5][19][20].
Exudate from chronic wounds such as pressure ulcers contains elevated levels of matrix metalloproteinases (MMPs), which can degrade the extracellular matrix, impairing cell migration and connective tissue deposition [21]. Growth factors are also inhibited by the MMPs found in chronic wound exudate, so the inflammatory phase often persists and the wound healing process does not progress to the proliferative phase [22].
The amount of exudate produced by pressure ulcers can be controlled by selecting the most appropriate absorptive dressing from a range of suitable dressings, such as hydrocellulars, hydrofibres, calcium-alginates, hydrocolloids, hydrogels and hydropolymers. The choice of dressing should reflect its ability to absorb excess exudate, minimise tissue trauma, debride devitalised tissue and remain in place. When managing skin sensitivity, it is also important to choose a hypoallergenic dressing. Moisture-retentive dressings have been associated with lower levels of reported pain during wound healing.
The ability to manage exudate in a controlled fashion is the key to obtaining optimal moisture balance at the wound interface, which reduces the risk of maceration and of the wound drying out. Maceration results from prolonged exposure of the skin to excessive moisture and is common around the margins of pressure ulcers. Prolonged contact with moisture also inhibits the skin's barrier function, increasing the risk of exogenous eczema and contact dermatitis [23]. Areas of erythema may be present where exudate is in contact with vulnerable skin, often leading to excoriation, the development of irritant dermatitis and new areas of tissue breakdown.
Dressings with adhesive borders should be avoided when treating patients with fragile, oedematous or macerated skin. Dressing performance may also be affected by the effects of friction and shear forces in areas such as the sacrum and heels, where dressings may ruck up and result in additional local pressure, leakage, maceration or skin sensitivity.
Incontinence dermatitis occurs as a result of high levels of moisture, bacterial and enzymatic activity, and the effects of friction. Erythematous maceration can be extremely painful so treatment should be prompt and may require the application of a topical corticosteroid for a limited time to reduce local inflammation before the application of a barrier preparation [24].
The maintenance of skin integrity is therefore an important factor in reducing the risk of tissue breakdown. Practice guidelines advocate that excess moisture as a result of incontinence, perspiration or exudate should be controlled by gently washing the skin with warm water immediately after soiling using mild cleansing agents, and that moisturisers be lightly applied to dry areas [3][5].
Grade 1 and 2 pressure ulcers are relatively superficial and heal primarily by epithelialisation (Figure 1). However, the presence of reactive hyperaemia, oedema, maceration, excoriation and blistering of the peri-ulcer skin can make it difficult to ascertain the true extent of tissue damage in this grade of pressure ulcer (Figure 2). Deeper wounds, such as grade 3 or 4 pressure ulcers, are characterised by full-thickness skin loss and often involve large cavities with undermining (Figure 3). These heal by secondary intention as the wound slowly fills in with granulation tissue, a process that depends on effective wound bed preparation. Epithelial advancement from the edge of these wounds is not the primary method of healing.
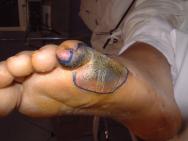
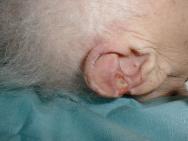
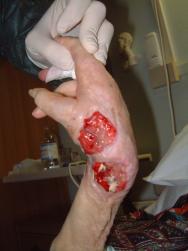
Wound healing is directly related to the size, shape and stage of the pressure ulcer, but the affected area does not reduce in a linear fashion. However, measuring grade 3 and 4 pressure ulcers at initial assessment and at regular but not too frequent intervals thereafter is a reliable predictor of outcome, and calculating healing rates has been shown to effectively distinguish between healing and non-healing ulcers [25][26][27]. In one study, all pressure ulcers that healed had a percentage area reduction of more than 47% at two weeks, which proved a reliable predictor of healing [25]. Best practice recommends that pressure ulcers that do not decrease in size within two weeks of active treatment should be re-assessed and treatment modified accordingly [28]. However, it should be noted that wound debridement can initially increase the size of a pressure ulcer.
Monitoring the wound size, condition of the surrounding skin and wound margin can provide a valuable indication of the efficacy of preventive treatment and should be carefully documented. The importance of accurately measuring pressure ulcers and assessing the skin is emphasised by the rising number of clinical negligence cases worldwide that involve pressure damage and have resulted in increasing claims for compensation [29].
Several pilot studies are evaluating the effectiveness of new technologies or therapies that aim to promote healing in pressure ulcers and reduce healing times. These include topical negative pressure (TNP) therapy and electromagnetic stimulation.
Also known as vacuum-assisted closure, this non-invasive, mechanical wound care treatment uses localised and controlled negative pressure (suction) to support and encourage the wound healing process. The negative pressure is applied in continuous or intermittent cycles via an evacuation tube connected to a computerised and programmable pump, with equal distribution to every surface of the wound ensured by a open-cell polyurethane foam dressing. This process:
provides a moist wound healing environment
reduces periwound oedema, which increases local blood perfusion
stimulates cell proliferation, accelerating the formation of granulation tissue [30][31].
These three factors encourage uniform epithelial migration and reduce the risk of infection. However, the delivery of TNP depends on the maintenance of an effective seal. This can be difficult when treating certain body areas, particularly sacral pressure ulcers that are continually subject to shear and friction.
Continuous therapy helps to remove fluid from the wound margins and stagnant fluid from within the wound. Oedema impairs healing by acting as a reservoir for infection and preventing adequate capillary blood flow to the wound bed, which also increases the risk of infection by limiting the availability of lymphocytes and macrophages [32]. Reducing periwound oedema decreases the bacterial burden, lowering the risk of colonisation, and enables fresh blood to penetrate the wound, stimulating the formation of granulation tissue.
Intermittent therapy acts as a mechanical stretch, resulting in the repeated release of the biochemical messengers that stimulate cellular proliferation and tissue growth, speeding up wound healing [33].
Wound size, tissue type, exudate level, odour and the condition of the surrounding skin should be monitored at each dressing change. TNP can be used to achieve complete healing or to prepare the wound bed for surgical closure, particularly as an alternative to traditional saline wet-to-moist dressings in chronic non-healing wounds of considerable depth. It can also be used to encourage the formation of granulation tissue and speed up healing rates in complicated chronic or non-healing wounds.
The presence of malignant lesions, untreated osteomyelitis and necrotic tissue within the wound bed reduce the effectiveness of negative pressure therapy and are contraindicated by the manufacturers. Caution should be exercised if there is bleeding, unstable local haemostasis or distal diabetic foot lesions, or if the patient has been prescribed anticoagulants. Contraindications include the use of TPN in wounds containing fistulae that communicate directly with organs or body cavities.
The electrical stimulation of electromagnetic fields has been used for years as an adjuvant therapy to treat pressure ulcers. The body has an endogenous bioelectrical system that can accelerate wound healing by attracting the different types of cells involved in the process to the wound bed. These cells carry either a positive or a negative charge and may be influenced by therapeutic levels of electrical current delivered to the wound from an external source [34]. Studies have shown that when compared with controls, both alternating and direct electrical currents produced a reduction in healing time, increased tissue perfusion and promoted collagen formation [35].
The Agency for Health Care Policy and Research (now the Agency for Healthcare Research and Quality) stated that electrical stimulation may be considered an adjuvant therapy for grade 1, 2 and 4 pressure ulcers. Data on the effectiveness of these adjuvant therapies in relation to wound healing has been encouraging, but studies suggest that different healing rates are produced with various electrotherapy modalities. Further controlled clinical trials are therefore necessary to establish the optimal parameters for the electrical stimulation of pressure ulcers.
Wound bed preparation is a relatively recent concept that is fast becoming established in the treatment of chronic wounds. The TIME framework presents a systematic approach to the local assessment and treatment of non-healing pressure ulcers, which make up the largest subset of chronic wounds in the developed world. The limitation of wound bed preparation is that the concept of TIME does not provide a useful framework for the wider aspects of patient management, including the identification of patients' at-risk status and pressure redistribution, which is of particular relevance when treating grade 3 and 4 pressure ulcers.
Advances in chronic wound management over the past decade have led to the development and implementation of a range of clinical guidelines on the prevention and treatment of pressure ulcers. The excellent cost-benefit relationship of these procedures has underlined the importance of pressure ulcer management in clinical practice.
1. European Wound Management Association. Position document: Wound bed preparation. London: MEP Ltd, 2004. Available from URL: http://www.ewma.org.
2. Clark M, Defloor T, Bours G. A pilot study of prevalence of pressure ulcers in European hospitals. In: Clark M, editor. Pressure Ulcers: Recent advances in tissue viability. Salisbury: Quay Books, 2004; 8-22.
3. European Pressure Ulcer Advisory Panel. Pressure Ulcer Treatment Guidlines. Oxford: EPUAP, 1999. Available from URL: http://www.epuap.org.
4. Anthony D, Reynolds T, Russell L. An investigation into the use of serum albumin in pressure sore prediction. J Adv Nurs 2000; 32(2): 359-65.
5. National Institute for Clinical Excellence. Inherited Clinical Guideline B: Pressure ulcer risk assessment and prevention. London: NICE, 2001. Available from URL: http://www.nice.org.uk.
6. European Pressure Ulcer Advisory Panel. Nutritional Guidlines for Pressure Ulcer Prevention and Treatment. Oxford: EPUAP, 2003. Available from URL: http://www.epuap.org.
7. Bishop SM, Walker M, Rogers AA, Chen WY. Importance of moisture balance at the wound-dressing interface. J Spinal Cord Med 2003; 12(4): 125-8.
8. Alvarez OM, Fernandez-Obregon A, Rogers RS. Chemical debridement of pressure ulcers: a prospective, randomized, comparative trial of collagenase and papain/urea formulations. Wounds 2000; 12(2): 15-25.
9. Sherman RA, Wyle F, Vulpe M. Maggot therapy for treating pressure ulcers in spinal cord injury patients. J Spinal Cord Med 1995; 18(2): 71-4.
10. Cox RA, Bowie PE. Methicillin-resistant Staphylococcus aureus colonization in nursing home residents: a prevalence study in Northamptonshire. J Hosp Infect 1999; 43(2): 115-22.
11. Dunford CE. Methicillin resistant Staphylococcus aureus. Nurs Stand 1997; 11(25): 58, 61-2.
12. Heggers JP. Defining infection in chronic wounds: methodology. J Wound Care 1998; 7(9): 452-6.
13. Gilchrist B. Taking a wound swab. Nurs Times 2000; 96(4 Suppl): 2.
14. Dow G. Bacterial swabs and the chronic wound: when, how, and what do they mean? Ostomy Wound Manage 2003; 49(5A Suppl): 8-13.
15. Romanelli M, Magliaro A, Mastronicola D, Siani S. Systemic antimicrobial therapies for pressure ulcers. Ostomy Wound Manage 2003; 49(5A Suppl): 25-9.
16. Lansdown AB. Silver. I: Its antibacterial properties and mechanism of action. J Wound Care 2002; 11(4): 125-30.
17. Cutting KF, White R. Defined and refined: criteria for identifying wound infection revisited. Br J Community Nurs 2004; 9(3): S6-15.
18. Parish LC, Witowski JA. The infected decubitus ulcer. Int J Dermatol 1989; 28(10): 643-7.
19. Brandeis GH, Ooi WL, Hossain M, Morris JN, Morris JN, Lipsitz LA. A longitudinal study of risk factors associated with the formation of pressure ulcers in nursing homes. J Am Geriatr Soc 1994; 42(4): 388-93.
20. Defloor T. The risk of pressure sores: a conceptual scheme. J Clin Nurs 1999; 8(2): 206-16.
21. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 2003; 200(4): 448-64.
22. Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen 1999; 7(6): 433-41.
23. Cameron J, Wilson C, Powell S, Cherry G, Ryan T. Contact dermatitis in leg ulcer patients. Ostomy Wound Manage 1992; 38(9): 10-1.
24. Newton H, Cameron J. Skin Care in Wound Management. A clinical education in wound management booklet. Holsworthy: Medical Communications UK Ltd, 2004.
25. van Rijswijk L. Full-thickness pressure ulcers: patient and wound healing characteristics. Decubitus 1993; 6(1): 16-21.
26. van Rijswijk L, Polansky M. Predictors of time to healing deep pressure ulcers. Ostomy Wound Manage 1994; 40(8): 40-2, 44, 46-8 passim.
27. Brown GS. Reporting outcomes for stage IV pressure ulcer healing: a proposal. Adv Skin Wound Care 2000; 13(6): 277-83.
28. Flanagan M. Wound measurement: can it help us to monitor progression to healing? J Wound Care 2003; 12(5): 189-94.
29. McKeeney L. Legal issues for the prevention of pressure ulcers. Journal of Community Nursing 2002; 16(7): 28-30.
30. Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997; 38(6): 553-62.
31. Philbeck TE, Whittington KT, Millsap MH, Briones RB, Wight DG, Schroeder WJ. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manage 1999; 45(11): 41-50.
32. Morykwas MJ, Argenta LC. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc 1997; 6(4): 279-88.
33. Fabian TS, Kaufman HJ, Lett ED, Thomas JB, Rawl DK, Lewis PL, et al. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg 2000; 66(12): 1136-43.
34. Pellett R. The use of electrical stimulation in wound healing: a review of the literature. Acute Care Perspectives 2000; 8(1): 9-15.
35. Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen 1999; 7(6): 495-503.