

|
Author(s)
R Gary Sibbald
Sylvie Meaume
Robert S Kirsner
Karl-Christian Münter
|
Contents
|
|
Published:
December 2005
Last updated: January 2006 Revision: 1.1 |
Keywords: Sustained-release silver foam dressing; evidence-based medicine; critically colonised wounds; cost-effectiveness.
There is a need for evidence to support the use of dressings containing silver in the management of critically colonised wounds.
In wound care, a new model needs to bridge the gap between the ideal and reality. This could be used to critically evaluate specific treatment procedures where there is only limited clinical and investigational evidence to support their use.
The use of an evidence-based medicine model can provide a framework for future analysis of the efficacy (clinical studies), efficiency (everyday practice) and effectiveness (relative cost) of new technologies in wound care.
This paper presents clinical evidence on a sustained-release silver foam dressing in chronic wounds with regard to efficacy, efficiency and effectiveness of evidence-based medicine. The results are derived from a randomised, controlled, clinical trial (efficacy), a real-life, randomised comparative study from everyday practice (efficiency), and a health-economic evaluation (effectiveness). The results indicate that this sustained-release silver foam dressing provides particular benefit for the treatment of critically colonised wounds. It is suggested that the use of the evidence-based medicine model may be a benchmark for a new level of clinical evidence in wound management.
Over the past few years there has been increased use of dressings containing silver, although there is only limited clinical and investigational evidence supporting specific treatment procedures. It has been suggested that silver dressings may be of particular benefit when used for the treatment of critically colonised wounds. This paper discusses the evidence base for a sustained-release silver foam dressing (Contreet Foam), focusing on three areas:
Clinical research - efficacy (clinical studies)
Outcomes research - efficiency (everyday practice)
Health-economic analyses - effectiveness (cost-effectiveness).
The results from a randomised, controlled clinical study including 109 patients are reviewed, as well as a comparative study from everyday practice involving more than 600 patients, and a health-economic analysis.
The highest level of evidence is required in order to practise evidence-based medicine[1]. This is particularly challenging in the field of wound care. Sackett (2000) defined evidence-based wound management as the integration of best research evidence with clinical expertise and patient values[2]. In practice, healthcare practitioners must review the best research evidence, and interpret and compare the research with the current methods of practice[1].
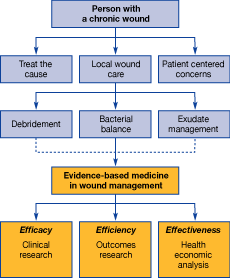
An assessment of the whole patient, the underlying cause, and any patient-centered concerns must be considered before examining the wound itself[3][4]. The concept of wound bed preparation includes debridement, control of bacteria and exudate management[3]. Wound bed preparation provides a framework to facilitate accurate diagnosis and treatment of patients with chronic wounds utilising holistic care and a team approach. The algorithm in Figure 1 identifies the components that should be considered in order to achieve the maximum benefit when using advanced wound care products [3][4]. In evidence-based medicine, efficacy, effectiveness and efficiency are three key concepts (see Box 1) [5]. These principles of evidence-based medicine are incorporated into this algorithm, emphasising that these issues should be integral to modern wound management.
All chronic wounds contain bacteria and the presence of bacteria obtained from a surface swab does not mean a wound is infected. The diagnosis of infection should be made clinically based on signs and symptoms of the local wound bed, the deeper structures and the surrounding skin. While clinicians have traditionally correlated bacteria number with outcomes, other factors are at play, including virulence and host resistance, as outlined in the equation [6]:

Host resistance is the ability of the host to resist bacterial invasion and the establishment of an increased bacterial burden or infection. There are systemic and local factors that can decrease host resistance. An increase in organism number and virulence of organisms causes the superficial wound bed to produce friable bright red granulation tissue, with an increased amount of slough on the surface, as well as increased discharge and odour. Decreased host resistance allows further bacterial proliferation and invasion of the organisms into deeper tissue.
Bacterial presence in a chronic wound can be described as a continuum starting with contamination or colonisation (bacterial balance) and ending with critical colonisation or infection (bacterial imbalance) as outlined in Figure 2.
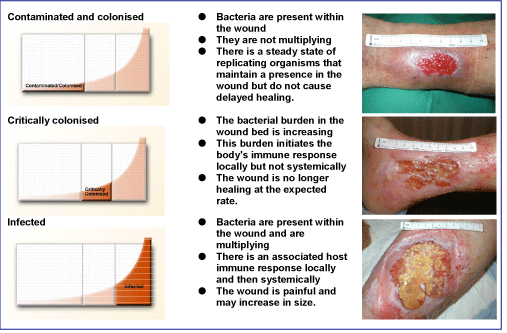
Some signs of critical colonisation in a wound are listed in Tables 1 and 2 . Chronic wounds change with time in a dynamic process and must be continuously re-evaluated and re-classified.
|
The signs described in Table 1 are localised in the superficial wound bed and are potentially treatable with topical agents, including ionised silver [7][8][9][10].
When host resistance is compromised, bacterial damage can extend beyond the local wound bed. More extensive bacterial damage results in a deeper and surrounding skin compartment infection that usually requires systemic antimicrobial treatment. The presence of surrounding skin pain, warmth and swelling with erythema and possible increase in wound size or new areas of satellite breakdown should alert the clinician to the possibility of a co-existing soft-tissue infection (cellulitis).
If the deep portion of the ulcer probes to bone, osteomyelitis is a possibility, especially if the patient is diabetic with neurotrophic foot ulceration [11]. With infection of the surrounding or deeper structures the wound often increases in size and there may be satellite areas of breakdown Table 2. At this stage, a bacterial swab can be used as a guide to treatment. It is necessary to make treatment decisions before the swab results are available. In general, wounds of less than one month's duration require treatment for Gram-positive organisms; however, if the wound has been present for more than one month, coverage for Gram-positive, Gram-negative and anaerobic organisms will be needed [12].
Superficial Topical therapy, for example silver dressings |
|
Deep and surrounding skin Systemic therapy |
|
The sustained-release silver foam dressing (Contreet Foam) comprises a soft hydrophilic polyurethane foam containing silver as an integral part of the dressing matrix [14]. The foam component provides a partial fluid lock to help prevent maceration of the surrounding wound margin by the absorbed wound fluid. Silver ions are present in a form that is readily hydro-activated in the presence of fluid or wound exudate [14]. The sustained-release silver foam dressing is active against a variety of micro-organisms including Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa and vancomycin-resistant enterococci (VRE). The sustained release of silver from this dressing has been demonstrated for up to seven days [15] [16].
The potential advantage of a foam and silver dressing combination is that surface bacterial counts can be reduced while removing excess exudate from the wound surface.
A clinical study reported elsewhere[17][18][19] was conducted comparing a sustained-release silver foam dressing with a foam dressing without silver (Allevyn Hydrocellular) in patients with venous or mixed venous/arterial leg ulcers and signs of stalled or delayed healing caused by suspected critical colonisation [9][20][21] . The primary aim of this study was to evaluate the relative reduction of ulcer size. The secondary aim was to assess wound-bed preparation. Bacterial balance was measured indirectly through a decrease in wound odour and the presence of healthy granulation tissue. Exudate management was measured through exudate leakage from the dressing and the absorption capacity of the dressing. The degree of maceration was also evaluated.
The study had a block randomisation, multi-centre comparative design. It was conducted in 15 centres predominantly in Europe, with two North American sites in Canada and the USA. There were 109 evaluable patients with chronic non-healing venous and mixed arterial and venous ulcers entered into the study. Fifty-two patients were randomised to the sustained-release silver foam dressing group and 57 patients to the foam dressing without silver group. The study was conducted for four weeks or until complete wound closure. The inclusion criteria comprised: an ankle/brachial index of 0.65 or higher, moderately to highly exudating wounds, delayed healing and treatment with compression therapy for four weeks prior to the study. Ulcers had to show signs of increased bacterial load characterised by one or more of the following: increased exudate, increased pain, discoloration of the granulation tissue and/or foul odour. Exclusion criteria included: the presence of clinical infection (deep or surrounding skin symptoms and signs) that required treatment with systemic antibiotics, and treatment with antibiotics or antiseptics for one week prior to inclusion.
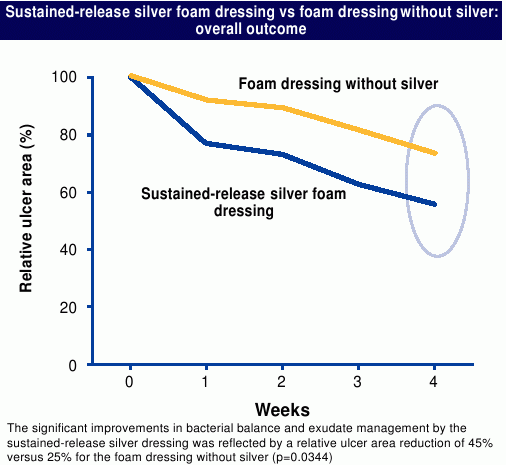
All patients were treated with appropriate compression therapy. After four weeks, the median relative reduction in ulcer area was 45% with the sustained-release silver foam dressing and 25% with the foam dressing without silver as shown in Figure 3 (p=0.0344, Wilcoxon two-sample test). There was a statistically significant difference in the reduction of wound odour in favour of the sustained-release silver foam dressing after one and four weeks (p=0.0013 and 0.0301, respectively, using the Wilcoxon two-sample test), while both groups demonstrated an increase in the presence of healthy granulation tissue.
At one week fluid leakage was noted in 27% of the sustained-release silver foam dressing changes compared with 44% of the foam alone group (p=0.06, Wilcoxon two-sample test). At the end of the study, there were significantly fewer dressing changes associated with exudate leakage in the sustained-release silver foam dressing group (19%) compared with the foam dressing without silver group (49%; p=0.002, Wilcoxon two-sample test). The sustained-release silver foam dressing had a significantly better absorption capacity compared with the foam dressing as evaluated on a five-point scale by study personnel at the end of treatment (p=0.04, Wilcoxon two-sample test).
The sustained-release silver foam dressing has also been evaluated in an effectiveness study in real-life settings. The Contreet Outcome Program (CONTOP) study, was conducted at more than 80 wound care centres in ten countries and has more than 600 patients enrolled. An interim analysis is reported on the first 352 patients with complete data available [22]. The endpoints in this study are the reduction in wound area, wound progress, exudate management, and patient-related outcomes. This study included a number of venous leg ulcers in addition to other aetiologies as shown in Table 3. Patients were randomised to receive either the sustained-release silver foam dressing or treatment in accordance with local best practice, that is the usual treatment provided by the clinic (see Table 4). The study duration was four weeks.
| Ulcer types | Frequency of ulcers | |
| Sustained-release silver foam dressing | Local best practice | |
| Venous leg ulcers | 43% | 48% |
| Mixed venous/arterial leg ulcers | 24% | 20% |
| Pressure ulcers | 7% | 7% |
| Diabetic foot ulcers | 9% | 5% |
| Other | 17% | 20% |
| Dressing type | Frequency |
| Foam/alginates | 45% |
| Hydrocolloid dressings/films | 15% |
| Gauze | 4% |
Antibiotic/antimicrobial
|
30% 14% (48%) 16% (52%) |
| Other | 6% |
The silver foam dressing decreased wound size by 50%, with the comparators reducing wound size by 30% (p=0.006, Wilcoxon two-sample test). There was also a statistically significant increase in the presence of normal granulation tissue in 68% of the sustained-release silver foam-treated patients and 50% of the local best practice group (p=0.002, Wilcoxon two-sample test). Exudate management was evaluated by dressing wear time: the sustained-release silver foam dressing group averaged 3.8 days, which was significantly longer than the local best practice group average of 2.3 days (p=0.0001, Wilcoxon two-sample test). The study also revealed that the sustained-release silver foam dressing showed statistically significant advantages in odour reduction and pain relief as well as the clinical ease of use of the dressing [22].
Traditionally the consideration of treatment costs in wound care has been limited to dressing costs alone. However, to properly evaluate the financial impact of a treatment strategy on a healthcare system, outcomes also need to be considered. In an analysis of the cost-effectiveness of using the sustained-release silver foam dressing the outcomes considered were average dressing wear-time, the healing time of the ulcers, cost of medical and nursing time for assessment and treatment, and the cost and frequency of complicating infections. A spreadsheet Figure 4 and a Markov model Figure 5[23][24] were designed to perform these health-economic analyses. The two health-economic models were based on methods and procedures from published cost-effectiveness models [23][24]. The analyses were performed with a societal perspective in a UK and German context. The model design and data were validated by a UK and German panel consisting of wound care experts. Following advice from the expert panels, the models were then adjusted and re-analysed and relevant sensitivity analyses were performed to assess the robustness of the models.
The analyses compared four different wound-care dressings used in the treatment of delayed healing venous leg ulcers. A four-week limit was applied in the main models for UK and Germany Figure 4. The Markov model assured that this four-week model had a realistic link to cost-effectiveness of complete wound closure. Figure 5 [25].
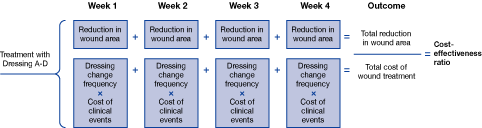
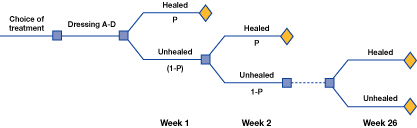
The costs of the dressings - and secondary dressings where needed - were determined and the weekly cost per dressing change was calculated using the data from Table 5 and Table 6 [25] [26]. The costs associated with materials including dressings, dressing change frequency, clinician time, and infection were compiled from relevant local sources [25] [26]. Costs specifically related to dressings are outlined in Table 6.
| Reduction in wound area per week (%) | Dressing changes per week | References | |
| Sustained-release silver foam dressing (Contreet Foam) | 12.6 | 2.2 | (Karlsmark et al 2003; Sibbald et al 2004) [14] [17] * |
| Sodium carboxymethylcellulose dressing containing silver (Aquacel Ag) | 6.0 | 1.9 | (Vanscheidt et al 2003) [27]) |
| Activated charcoal cloth with silver dressing (Actisorb Silver 220) | 11.2 | 3.6 | (Tebbe and Orfanos 1996) [28] |
| Cadexomer iodine paste (Iodoflex) | 9.0 | 2.7 | (Hansson et al 1998) [29] |
| Cost per dressing (euro) | ||||||
| United Kingdom | Germany | |||||
| Primary | Secondary | Total | Primary | Secondary | Total | |
| Sustained-release silver foam dressing (Contreet Foam) | 10.43 | Not necessary* | 10.43 | 11.5 | Not necessary* | 11.5 |
| Sodium carboxymethyl-cellulose dressing containing silver (Aquacel Ag) | 5.93 | 2.91 (non-adhesive, absorbent: CombiDERM-N) | 8.84 | 7.26 | 14.2 (self-adhesive, carboxymethoyl-cellulose island dressing:Versiva) | 21.46 |
| Activated charcoal cloth with silver dressing (Actisorb Silver 220) | 3.53 | 4.32 (non-adhesive, hydropolymer foam dressing: Tielle Plus Borderless) | 7.85 | 5.46 | 10.33 (non-adhesive, hydropolymer foam dressing: Tielle Plus Bordeless) | 15.79 |
| Cadexomer iodine paste (Iodoflex)** | 14.04 | 0.48 (knitted viscose, silicone impregnated non-adherent dressing: N-A Dressing) | 14.52 | N/A | N/A | N/A |
* Contreet Foam does not need a secondary dressing. ** Iodoflex is not part of the German models, as it is not available in Germany. The weekly cost per dressing change was calculated as: Weekly dressing change cost = (Primary and secondary dressing cost + Nursing time + Travel cost + Gloves + Wound cleansing + Compression bandages) x Dressing changes per week x The cost of risk of infection. The choice of secondary dressings was based on manufacturers' instructions for use and the expert panels' advice. The size of the secondary dressing was selected so it could fully cover the primary dressing. For the sensitivity analysis, cheaper alternatives were selected, again based on the expert panels' advice.
The results from the weekly cost per dressing change were used to calculate the cost per percentage reduction in wound area as shown in Table 7[25][26].
| Cost per percentage relative reduction in wound area (euro) | ||
| United Kingdom | Germany | |
| Sustained-release silver foam dressing (Contreet Foam) | 14.18 | 4.18 |
| Sodium carboxymethyl-cellulose dressing containing silver (Aquacel Ag) | 26.37 | 11.10 |
| Activated charcoal cloth with silver dressing (Actisorb Silver 220) | 24.81 | 8.90 |
| Cadexomer iodine paste (Iodoflex) | 25.43 | NA |
The differences in costs between UK and German results are primarily due to different cost structures for nursing salaries in the two countries. [25][26].
Sensitivity analyses were performed to assess the robustness of the results. Both the cost and efficacy data were altered up and down by 20%. These calculations also included replacing the secondary dressings with less costly alternative dressings where appropriate. The link between cost of relative reduction in wound area and the cost per healed wound was ensured through the use of the Markov model. The results from this Markov model confirmed the results from the four-week model [25]. The sensitivity analyses showed that the models were robust and that the sustained-release silver foam dressing was the most cost-effective choice in regard to cost per relative reduction in wound area as well as cost per healed wound [25].
The importance of the bacterial load, the appearance of the superficial wound bed including wound exudate, the host resistance, and the most appropriate course of treatment for patients with critically colonised and infected wounds are subject to debate and disagreement. The importance of evidence-based studies to support optimal decision making for wound treatment cannot, therefore, be overstated.
This review article summarises the use of a sustained-release silver foam dressing in the treatment of non-healing chronic wounds. Roma-Moore stated that 'only controlled clinical evaluations ideally support final decisions about the best treatments to use in achieving wound care outcomes'[30]. The evidence-based medicine approach combined with the principles of wound bed preparation led to the design of the first RCT of a moist wound healing silver dressing for chronic wound care.
In this RCT, a sustained-release silver foam dressing significantly decreased odour and exudate faster than the non-active foam dressing. The results showed a 45% reduction in ulcer size from baseline for the sustained-release silver foam dressing compared with 25% for the foam dressing without silver. In a recent study by Vanscheidt et al evaluating a sodium carboxymethylcellulose dressing containing silver (Aquacel Ag) the mean ulcer area was reduced by 23.9% over the same period of time (four weeks) [27]. According to Flanagan (2003) a percentage area reduction of less than 20-40% over the initial two to four weeks of treatment is a reliable indicator that the wound is not responding well to the treatment [31].
Randomised, controlled trials are often criticised because they do not represent the full spectrum of patients in real-life settings. The CONTOP study was designed to address the issues of everyday practice and has produced comparable results to the randomised, controlled trial of the benefits of the sustained-release silver foam dressing compared with local best practice. The results showed a 50% reduction in ulcer size for the sustained-release silver foam dressing compared with 30% for local best practice. In addition, the dressings were changed less frequently in patients treated with the sustained-release silver foam dressing.
New products will not become part of everyday practice if they add additional costs to the healthcare system. By calculating the total healthcare costs based on evidence and expert opinion as developed in the models outlined in this paper, the sustained-release silver foam dressing was demonstrated to provide cost-effective treatment of wounds both in terms of cost per percentage relative reduction in wound area and cost per healed wound. This is a preliminary estimate of real clinical costing of the components that are included in cost-effectiveness analyses. This concept needs to be prospectively tested in practice and, depending on the clinical expertise, the healthcare system obstacles and the cost of the product, these figures can change and need to be critically evaluated for each healthcare delivery model.
The results of this analysis indicate that the sustained-release silver foam dressing (Contreet Foam) is of particular benefit for the treatment of critically colonised wounds. The use of an evidence-based medicine model provides a framework for future analysis of the efficacy, efficiency and effectiveness of new technologies in wound care. This model adds rigour and sets a benchmark for a new level of clinical evidence. This is of particular importance in areas of wound management where there is only limited clinical and investigational evidence to support specific treatment procedures.
1. Ryan S, Perrier L, Sibbald RG. Searching for evidence-based medicine in wound care: an introduction. Ostomy Wound Manage 2003; 49(11): 67-75.
2. Sackett DL. The fall of 'clinical research' and the rise of 'clinical-practice research'. Clin Invest Med 2000; 23(6): 379-81. Erratum in Clin Invest Med 2001; 24(1): 4.
3. Sibbald RG, Williamson D, Orsted HL, Campbell K, Keast D, Krasner D, et al. Preparing the wound bed - debridement, bacterial balance, and moisture balance. Ostomy Wound Manage 2000; 46(11): 14-35.
4. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D; International Wound Bed Preparation Advisory Board; Canadian Chronic Wound Advisory Board. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003; 49(11): 23-51.
5. Price P. The challenge of outcome measures in chronic wounds. J Wound Care 1999; 8(6): 306-8.
6. Dow B, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage 1999; 45(8): 23-27, 29-40.
7. Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen 2001; 9(3): 178-86.
8. Gardner SE, Frantz RA, Troia C, Eastman S, MacDonald M, Buresh K, et al. A tool to assess clinical signs and symptoms of localized infection in chronic wounds: development and reliability. Ostomy Wound Manage 2001; 47(1): 40-7.
9. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care 1994; 3(4): 198-201.
10. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003; 11(Suppl 1): S1-S28.
11. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995; 273(9): 721-3.
12. Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen 2000; 8(5): 347-52.
13. Sibbald RG, Browne AC, Coutts P, Queen D. Screening evaluation of an ionized nanocrystalline silver dressing in chronic wound care. Ostomy Wound Manage 2001; 47(10): 38-43.
14. Karlsmark T, Agerslev RH, Bendz SH, Larsen JR, Roed-Petersen J, Andersen KE. Clinical performance of a new silver dressing, Contreet Foam, for chronic exuding venous leg ulcers. J Wound Care 2003; 12(9): 351-4.
15. Lansdown AB, Jensen K, Jensen MQ. Contreet Foam and Contreet Hydrocolloid: an insight into two new silver-containing dressings. J Wound Care 2003; 12(6): 205-10.
16. Dolmer M, Larsen K, Jensen M. In vitro silver release profiles for various antimicrobial dressings. Poster presented at the 2nd World Union of Wound Healing Societies Meeting, July 2004; Paris.
17. Sibbald RG, Jørgensen B, Lohmann M, Harding KG, Price P, Gottrup F, et al. Wound bed preparation: properties of a foam dressing and a silver-containing foam dressing. Poster presented at the 2nd World Union of Wound Healing Societies Meeting, July 2004; Paris.
18. Price P, Jørgensen B, Lohmann M, Gottrup F, Andersen KE, Bech-Thomsen N, et al. Health-related quality of life aspects after treatment with a foam dressing and a silver-containing foam dressing in chronic leg ulcers. Poster presented at the 2nd World Union of Wound Healing Societies Meeting, July 2004; Paris.
19. Jørgensen B, Price B, Andersen KE, Gottrup F. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonized venous leg ulcers: a randomised, controlled trial. Int Wound J 2005; 2(1): 64-73.
20. Kirsner R. Wound bed preparation. Ostomy Wound Manage 2003; Feb(Suppl): 2-3.
21. Enoch S, Harding K. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003; 15(7): 213-29.
22. Russell L, Nebbioso G, Münter KC, Beele H, Basse PB. The CONTOP study: A hydro-activated silver-containing foam dressing versus standard care. Poster presented at the 2nd World Union of Wound Healing Societies Meeting, July 2004; Paris.
23. Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998; 13(4): 397-409.
24. Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13(4): 322-38.
25. Scanlon E, Münter KC, Hart-Hansen K. Cost-effectiveness of a silver-containing hydro-activated foam dressing in Germany and the UK. Poster presented at the 2nd World Union of Wound Healing Societies Meeting, July 2004; Paris.
26. Münter KC, Sellemer W, Poulsen PG, Hahn TW, Metzler B. Gesundheitsökonomische Analyse über die Anwendung eines silberhaltigen Schaumverbandes bei venösen Ulcera cruris mit verzögerter Wundheilung. Poster presented at the 7. Kongress der Deutschen Gesellschaft für Wundheilung und Wundbehandlung eV. (DGFW), 2003; Augsburg.
27. Vanscheidt W, Lazareth I, Routkovsky-Norval C. Safety evaluation of a new ionic silver dressing in the management of chronic ulcers. Wounds 2003; 15(11): 371-78.
28. Tebbe C, Orfanos CE. Behandlung von ulcera cruris und dekubitus mit einem xerodressing: phasenübergreifende wundauflage mit antimikrobieller wirksamkeit. Zeitschrift für Hautkrankenheiten (H+G Special Edition) 1996; 71: 697-702.
29. Hansson C. The effects of cadexomer iodine paste in the treatment of venous leg ulcers compared with hydrocolloid dressing and paraffin gauze dressing. Cadexomer Iodine Study Group. Int J Dermatol 1998; 37(5): 390-6.
30. Roma-Moore A. Controlled clinical evaluations vs case studies: why wound care professionals need to know the difference. In: Krasner DL, Rodeheaver GT, Sibbald RG, editors. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals,. Third Edition. Wayne, PA: Health Management Publications Inc, 2001.
31. Flanagan M. Wound measurement: can it help us to monitor progression to healing? J Wound Care 2003; 12(5): 189-94.