

|
Author(s)
Shyam Rithalia Reader
Laurence Kenney Research
Fellow
|
Contents
|
|
Published:
September
2001
Last updated: September 2001 Revision: 1.0 |
Keywords: pressure ulcers; assessment techniques; support surfaces; interface pressure; pressure relief index.
The science of support surface evaluation is still at an early stage.
The most commonly used quantitative technique is the measurement of interface pressure. Other parameters measured include interface temperature and humidity and tissue perfusion. All of these measures should be treated carefully when selecting a support surface.
The mechanisms underlying the aetiology of pressure ulcers are not fully understood and therefore single physical measures are likely to be poor predictors of outcome.
Better quality clinical trial data is becoming available, but the results have yet to be linked to the outcomes of quantitative studies on the same support surfaces.
Evaluation remains more of an art than a science.
Pressure ulcers cause great pain and suffering to patients and their treatment is both costly and time consuming. Therefore every effort should be directed towards their prevention. The mechanisms underlying the aetiology of pressure ulcers are complex and multi-dimensional and are not fully understood. Accurate assessment of pressure relieving devices aimed at prevention is difficult. Over the years, numerous parameters, including interface pressure and transcutaneous blood gas measurements have been used to evaluate mattresses and cushions. All of the approaches used are, at best, limited in themselves and require cautious interpretation. The science of evaluation of support surfaces would appear to be still at a formative stage, with clinical validation of many of the approaches yet to be carried out.
Over the past few years, there has been a considerable increase in both the variety and number of patient support surfaces [1], [2]. The cost of these devices range from a few hundred to several thousand pounds and hence the importance of a logical approach to their selection cannot be overstated. Inappropriate selection not only wastes capital resources, but it can also be detrimental to the patient. However, despite recent trends in the UK towards basing clinical decisions on evidence from randomised controlled trials [3], the selection of support surfaces remains a relatively neglected area, with little solid theoretical evidence and few properly controlled clinical trials [4], [5], [6].
Evaluation decisions still need to be made and those required to select support surfaces are faced with a rather confusing and often misleading array of commercial literature. For example, suppliers' literature includes phrases such as "It allows fluid to drain, air to circulate and actually uses redistributed pressure to maintain the flow of oxygen and nutrients to body tissue", or "The cover is designed to achieve pressure elimination." Statements such as these are at best unsubstantiated and, in some cases, factually incorrect. There are also examples in the literature where authors of various reports and articles have unwittingly supported such statements [7], [8], [9].
The literature published in the technical journals on mattress assessment may also prove daunting. These difficulties arise from the complex nature of tissue mechanics and body-support surface interface modelling, together with a still incomplete understanding of the aetiology of pressure ulcers. Researchers have therefore developed experimental methods of assessment (Figure 1, Table 1) based upon the existing body of evidence [10] and the use of available sensor technology.
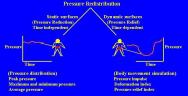
It is widely accepted that in vulnerable patient groups, prolonged tissue distortion resulting from localised pressure and/or shear forces will result in tissue damage [11], [12]. The magnitude, duration and direction of loading are considered to be the main causative factors leading to skin lesion formation [13], [14].
One mechanical parameter that is commonly measured and quoted in the literature on support surfaces is interface pressure (IP) or tissue contact pressure. IP is the pressure measured over a small area, resulting from applied loads, predominantly a patient's weight. It is a scalar quantity, providing information of magnitude but not direction, and represents a measure of the forces normal to the surface being transmitted through the area of the sensor in contact with both the body and the support surface. Pressure does not provide sufficient information to fully describe the loading at the particular point on the body surface and cannot be used in isolation to infer internal stresses and strains in the tissue. Due to the unavailability of suitable transducers, experimental shear force data is lacking in the literature [15].
IP is determined by various factors, including posture and the local shape and stiffness of both the tissue and support surface. Therefore, it is highly debatable whether simple interpretation of IP readings, such as pressure averaged over a number of sensors, should be used to select a support surface. Nevertheless, localised high IP readings do suggest high levels of tissue distortion which can lead to capillary occlusion and disruption of the lymphatic system [11], [16]. If this effect continues over a sufficient period of time, it will result in tissue damage [10], [17].
Apart from the mechanical parameters, time is also of significant importance and several investigators have established an inverse relationship between the intensity and duration of pressure to produce tissue damage [18]. Experimental studies on animals have shown that localised low pressure maintained for long periods can be as traumatic as localised high pressure maintained over short periods [18].
The testing of the effectiveness of a mattress or cushion in reducing IP levels has usually been carried out in young and healthy subjects, as patients on whom investigators would most like to measure various parameters are the least able to tolerate the measurements. It is likely that the subject characteristics, such as tissue and local joint stiffnesses affects absolute IP values. However, when comparing results between tests on different mattresses this factor may well not be significant, as all the results will have been derived using similar volunteers.
It is easy to demonstrate that the equipment used to measure IP produces an accurate reading under specific laboratory controlled conditions, namely between flat surfaces and under controlled temperature [19]. However, in measurements between the support surface and the body, these conditions are often not met and difficulties can arise.
For sensors located between the body and support surface, great care has to be taken in their selection and application. For example, individual transducers must be smaller in size than the area of interest, as well as of negligible stiffness and thickness so as not to interfere with the parameters being measured. With some commonly used devices, notably pressure mats, it is difficult to accurately locate and describe the location of the transducer relative to the body site [20].
When the sensor is placed between the body and the support surface, validation of measurements is difficult. It is interesting to note that around the at-risk areas where the pressure gradient is high, readings are likely to show the highest variability due to both the high sensitivity to small variations in sensor placement and problems with distortion of readings by local curvatures of the body-support surface interface.
Although there are many publications which present and interpret IP, there has been little scientific validation work, and the work that has been carried out has raised some worrying issues, such as the lack of repeatibility following re-positioning of a patient[21], [22]. A small number of researchers are addressing this, for example with the use of a standardised model-based test [23].
Since the validity of many of the published results remains in some doubt, it is probably true to say that the value of decisions based entirely on pressure readings provided by the suppliers, especially mean values, are questionable.
Maximum (peak) and minimum pressure readings under the bony tuberosities of the body are often reported in investigations or marketing brochures. Although this approach has some validity for static surfaces, whose mechanical characteristics do not change with time, it does not provide an accurate indication of the performance of a dynamic surface or reflect recovery time. In an extreme case, as shown in Figure 2, the measurement of maximum and minimum pressures may give an entirely misleading impression of the ability of an alternating system to relieve pressure for a significant period of time. Therefore, the pressure relieving power of an alternating pressure mattress (APAM) or cushion (APAC) cannot be predicted solely from maximum and minimum IP.
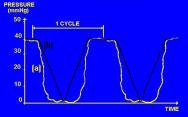
Average interface pressure is a highly abstracted and dubious value even for static surfaces, as it relies on the exact number and placements of sensors. More alarmingly, average IP has been used to compare the potential performance of APAMs with static devices [24], [25]. This approach has been described as meaningless [26] as it does not communicate the extent to which pressure is periodically relieved when using a dynamic surface.
The implicit recognition that tissue damage is a time dependent process underpins the traditional nursing approach of regularly turning the patient and more recently, the development of APAMs. For static mattresses, the time factor is impossible to accommodate as changes to pressure distribution can only come from movement of the patient. However, for dynamic surfaces, where the mechanical properties change with time in a predetermined manner, time should be included in any assessment technique.
A parameter used in the assessment of dynamic surfaces is accumulated pressure, called the pressure impulse, which was first described over 15 years ago by Bennett and Lee [27]. The pressure impulse is calculated as the area under the IP-time curve. Selective use of this technique has been reported by Clark [28]. However, the parameter is measured in units that are rather abstract and not easy to relate to clinical practice (mmHg/hr) and provides no clear indication of pressure-free time or the extent of pressure removal achieved. A more recent method for communicating both IP and time in a straightforward manner is the Pressure Relief Index (PRI) [29], [30], [31].
The PRI is an alternative and more recent technique for the assessment of alternating pressure mattresses and cushions [29], [30], [31]. This technique assesses the ability of an APAM to sustain IP below a chosen set of thresholds. These may represent thresholds thought to be clinically relevant, such as mean arteriolar (approximately 30 mmHg), capillary (approximately 20 mmHg) and venule (approximately 10 mmHg) operating pressures, or some other set of pressures which have meaning for the clinician intent on selecting a support surface [32].
The pressure time characteristics are analysed by a computerised system (Figure 3), which records the air pressure and interface pressure (Figure 4) of an alternating air pressure mattress or cushion over time. Horizontal lines are drawn at the IP thresholds of interest, and the time the APAM-IP traces spend below these lines is measured (Figure 5).
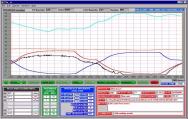
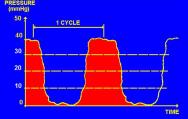
PRI is a non-dimensional ratio representing the proportion of time IP, measured at a particular location, below a specific threshold within a single cycle. Over one complete alternating cycle, it is possible to determine how many minutes or seconds the IPs remained below these thresholds. This gives an index of the recovery time allowed below a given IP for all varieties of cycle, cell inflation and sequence.
Performance measurements such as PRI can be very useful when looking at differences between APAMs, but can only indicate relative merit with respect to pressure relief. The technique requires expensive computerised equipment.
The occlusion of the blood supply to and lymphatic drainage from the tissue as a consequence of high interface loads can cause tissue damage. Therefore, it would seem sensible to measure these parameters directly, rather than rely on indirect and usually incomplete measures of the mechanical loads at the interface.
There has been increasing interest in the assessment of skin tissue perfusion by heated transcutaneous oxygen (tcPO2) and carbon dioxide (tcPCO2) sensors [33], [34]. Transcutaneous gas monitoring was originally developed for the measurement of arterial blood PO2 (PaO2) and PCO2 in neonates [35], [36]. Recently, this method has been used for the comparison of different support surfaces [37]. The sensors are applied directly to the skin and consist of a miniaturised polarographic Clark type electrode [38] and Stow-Severinghaus electrode [39]. A heating element is incorporated for regulating the temperature under the membrane, usually to 44°C. The measurements are influenced by many other factors including skin circulation under the electrode [40], [41].
Although transcutaneous measurements are reliable, simple and sensitive to the local variations in microcirculation, the size of the sensor is a problem; it can give false readings unless the influence of direct pressure on to the electrode is diffused over a large area. Moreover, there is some difficulty in attaching any importance to low tcPO2 values (0-35 mmHg), as this does not preclude tissue survival at the site of measurement [42]. Some examples [43], [44] of the use of PRI and transcutaneous gas tension readings are given in Table 2 and Table 3.
Other methods of assessing tissue blood supply include the use of laser Doppler fluxmetry [45], [46] and plethysmography [47]. These techniques have been used in laboratory-based studies on the effect of pressure on microcirculation, but as yet have not been successfully used as a tool for the assessment of support surfaces.
A positive relationship between local blood flow and skin temperature has been established by several investigators [48], [49]. Even a small rise in temperature of 1°C can increase metabolic activity and oxygen demand by 10 per cent, while heat loss may cause vasoconstriction and discomfort to the patient [50], [51]. Changes in skin temperature are a valuable guide to the condition of tissue subjected to localised pressure and ischaemia [52]. Excessive sweating or moisture between the skin and support surface increases the risk of pressure ulceration as friction forces are increased in the presence of moisture [53], [54]. Clinical experience has also shown the tendency of skin to macerate when it remains in a very humid environment for long periods of time.
It is possible to investigate the tissue response to ischaemic insult at bony prominences by skin surface temperature and humidity monitoring. This approach has been used by several investigators [50], [55] to quantify tissue distress resulting from prolonged application of compressive loads [51]. Both temperature and humidity are influenced by several factors within the body and the environment including length of time sitting on the seat, air convection, heat radiation from lights or other sources, hormonal variations and medication. However, the interpretation of local temperature and humidity findings is not straightforward and it takes much experience before either method can be used reliably.
In theory, the best evidence upon which to base the selection of pressure relief devices in reducing the incidence of ulcers is the randomised controlled trial (RCT). Matching patients for age, sex and other general conditions by standardising on one of the many risk scoring systems [56], [57] can enhance the reliability of the results.
However, clinical trials using mattresses are difficult to design and execute, while the increased interest and enthusiasm in both staff and patients involved can often influence the results [6]. Evaluation of treatment outcomes can be weak and there is also the problem of the variety of pressure sore risk scoring systems [57] in use, which makes it very difficult to compare the results obtained by other investigators.
A recent Cochrane review [4] concluded that "there was sufficient evidence from RCTs to show that patients at high risk of pressure sore development should not be placed on ordinary foam mattresses and that certain 'high specification foams' had been shown to be significantly better in terms of prevention. However, there was insufficient RCT-based evidence to clarify the relative merits of higher-tech constant low pressure and alternating pressure mattresses. Seat cushions have not been adequately evaluated." An update to the Cochrane Review, the Heath Technology Assessment series on wound care, entitled "Pressure-relieving beds, mattresses and cushions for the prevention and treatment of pressure sores" arrives at similar conclusions [6].
There are many unresolved problems in the assessment of different support surfaces associated with the measurement techniques and interpretation of results. Misconceptions, particularly amongst the nurse-based literature are common and there is confusion over assessment techniques for static and APAM mattresses.
Other notable and more fundamental limitations are the absence of sensors to adequately measure the complete set of forces acting on a particular point and the difficulties experienced with interpreting the incomplete data provided by IP readings in terms of tissue stresses and strains. For example, experimental data have been provided by researchers [58], [59], which shows higher pressures at the bone and soft tissue interface compared to IP values at the skin. Tissue mechanics is a highly complex and difficult area, but despite the many limitations of IP as an assessment parameter, measurements do provide some form of comparative data regarding the relative effectiveness of different surfaces. There remains, however, a lack of good quality clinical data to support the engineering-based evaluation criteria [4], [5], [6].
Assessment techniques are improving and further work from engineers, physiologists, nurses and others clinicians, to both improve and perhaps more importantly, validate, existing techniques, will help the assessment process to become less of an art and more of a scientific method.
Pressure ulcers should be less of a problem if preventive measures for at-risk patients are instituted at an early stage. Decisions on the allocation of the most appropriate support surface for a particular patient will be made easier with the development of improved risk assessment scoring systems. Advances in this area will not only emerge through the refinement of existing qualitative approaches, such as the Waterlow scoring system [56], but also through the introduction of more quantitative measures of risk assessment. Recent work in this area has investigated tissue electrical impedance properties as a discriminator of pressure ulcer risk [60].
A review of the literature [61], [62], [63] shows that about 80 per cent of sores occur in the four anatomical positions, namely the sacrum, ischium, trochanter and heel. Their relative distribution could be considered as a weighting to IP values. When giving overall pressure relief performance figures, it is conceivable to give the IP readings a higher weighting for a site that has a higher incidence of pressure ulcers. Factors, such as skin surface tension, shearing force, temperature and humidity, in addition to the magnitude and the duration of uniaxial load or interface pressure should be evaluated. Any evaluation procedure should also include patient comfort factor, which to date has received little attention. It is clear that a set of guidelines or protocol is needed for the evaluation of patient support surfaces for the prevention of pressure ulcers.
| Methods | Limitations |
| Maximum and minimum pressure[64][65] | Gives no indication of the time varying nature of the pressure |
| Average pressure[26][66] | Does not communicate the extent to which pressure is periodically relieved |
| Tissue deformation index[67] | A large number of measurements are required and it is complex to calculate |
| Pressure impulse[27] | Uses non-intuitive units of measurement (mmHg/h) and provides no clear idea of pressure-free time |
| Pressure relief index[29][30] | Requires expensive computerised equipment and is useful in looking at alternating systems only |
| Skin gas tension[40][68] | False readings occur, unless the influence of direct pressure on to the electrode is diffused |
| Comfort and quality of sleep[69][70] | Highly subjective and can be difficult to measure and analyse |
| Skin temperature and humidity[71] | Both techniques are influenced by several factors within the body and the environment |
| Clinical trials[4][5][6][72] | Can be influenced by the increased interest and enthusiasm of staff and patients involved |
| Alpha X cell | Astec 345 | Biwave Plus | PPS 2000 | |||||||||
| Observation | Mean | ąSD | Range | Mean | ąSD | Range | Mean | ąSD | Range | Mean | ąSD | Range |
| Baseline tcPO2 (mmHg) | 82.9 | 6.1 | 73-88 | 82.1 | 7.4 | 67-89 | 83.5 | 6.4 | 73-88 | 82.5 | 7.7 | 74-87 |
| Baseline tcPC02 (mmHg) | 41.9 | 3.1 | 45-38 | 41.5 | 2.5 | 39-44 | 41.3 | 2.2 | 38-44 | 41.4 | 2.6 | 39-43 |
| Lowest tcPO2 (mmHg) | 59.2 | 14.4 | 24-76 | 69.1 | 6.6 | 56-78 | 57.1 | 9.5 | 44-72 | 57.7 | 9.4 | 42-74 |
| Highest tcPCO2 (mmHg) | 44.9 | 2.7 | 40-49 | 44.4 | 2.4 | 41-49 | 46.6 | 4.0 | 40-55 | 48.0 | 5.8 | 41-61 |
| Area under tcPO2 curve (units/h) | 687.5 | 311.3 | 400-1376 | 509.4 | 179.4 | 313-769 | 809.8 | 292.7 | 529-1521 | 859.4 | 189.7 | 653-1193 |
| Area under tcPCO2 curve (units/h) | 132.8 | 63.9 | 44-237 | 138.9 | 73.1 | 41-289 | 150.9 | 89.8 | 55-304 | 207.8 | 111.1 | 86-398 |
| PRI <10mmHg (min/h) | 54.5 | 6.8 | 40-60 | 55.5 | 5.2 | 47-60 | 34.5 | 8.6 | 24-58 | 33.3 | 14.6 | 17-60 |
| PRI <20mmHg (min/h) | 42.4 | 5.3 | 32-53 | 38.3 | 8.3 | 27-59 | 28.9 | 5.0 | 22-44 | 18.9 | 4.5 | 11-27 |
| PRI <30mmHg | 28.1 | 6.9 | 16-44 | 25.0 | 5.5 | 17-37 | 23.2 | 4.3 | 11-29 | 8.7 | 6.9 | 0-21 |
| Airwave mattress | Nimbus 2 mattress | p values | ||||||||||
| Observations | Mean | ą SD | Range | Mean | ą SD | Range | ||||||
| Maximum IP | 40.1 | 8.3 | 25-54 | 25.8 | 3.6 | 20-32 | 0.0006* | |||||
| Minimum IP | 0.8 | 1.4 | 0-4 | 0.8 | 1.3 | 0-3 | 0.8760 | |||||
| Peak air pressure | 49.9 | 4.1 | 47-54 | 27.9 | 2.5 | 26-31 | <0.0001* | |||||
| PRI <10mmHg (min/h) | 13.3 | 2.0 | 10-15 | 25.8 | 2.8 | 23-33 | 0.0019 | |||||
| PRI <20mmHg (min/h) | 18.3 | 5.0 | 12-27 | 41.9 | 5.0 | 32-48 | <0.0001* | |||||
| PRI <30mmHg (min/h) | 34.3 | 15.3 | 14-60 | 57.6 | 5.6 | 43-60 | <0.0001* | |||||
| Baseline tcPO2 | 83.7 | 6.3 | 72-89 | 82.1 | 8.0 | 66-89 | 0.1427 | |||||
| Baseline tcPCO2 | 42.2 | 2.7 | 38-46 | 41.6 | 2.4 | 38-44 | 0.1934 | |||||
| Lowest tcPO2 | 63.3 | 6.4 | 58-73 | 70.1 | 6.1 | 54-78 | 0.0024* | |||||
| Highest tcPCO2 | 45.8 | 2.4 | 41-50 | 44.7 | 2.3 | 41-48 | 0.1039 | |||||
| Area under tcPO2 curve | 769.4 | 257.1 | 484-1241 | 552.5 | 219.6 | 240-967 | 0.0034* | |||||
| Area under tcPCO2 curve | 122.2 | 40.2 | 57-169 | 145.6 | 56.4 | 56-175 | 0.3379 | |||||
| * Statistically significant difference at p<0.05 | ||||||||||||
1. Lockyer-Stevens NA. Developing information base for purchasing decisions: a review of pressure-relieving beds for at-risk patients. Professional Nurse 1994; 9: 534-42.
2. Gebhardt KS, Bliss MR, Winwright PL, Thomas J. Pressure-relieving supports in an ICU. J Wound Care 1996; 5(3): 116-21.
3. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ 1996; 312(7023): 71-2.
4. Cullum N, Deeks J, Sheldon TA, Song F, Fletcher AW. Beds, mattresses and cushions for pressure sore prevention and treatment. Cochrane Database Syst Rev 2000; (2): CD001735.
5. Thomas DR. Issues and dilemmas in the prevention and treatment of pressure ulcers: a review. J Gerontol A Biol Sci Med Sci 2001; 56(6): M328-40.
6. Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess 2001; 5(9): 1-221.
7. Hampton S. Evaluation of the new Cairwave Therapy System in one hospital trust. Br J Nurs 1997; 6(3): 167-70.
8. Hibbs P. Tissue viability. Action against pressure sores. Nurs Times 1988; 84(13): 68-73.
9. Livesley B. Pressure sores. Airwaves take the pressure. Nurs Times 1986; 82(32): 67-71.
10. Bliss MR. Aetiology of pressure sores. Reviews in Clinical Gerontology 1993; 3: 379-97.
11. Brienza DM, Karg PE, Brubaker CE. Seat cushion design for elderly wheelchair users based on minimization of soft tissue deformation using stiffness and pressure measurements. IEEE Trans Rehabil Eng 1996; 4(4): 320-7.
12. Dinsdale SM. Decubitus ulcers: role of pressure and friction in causation. Arch Phys Med Rehabil 1974; 55(4): 147-52.
13. Reichel SM. Shearing force as a factor in decubitus ulcers in paralegics. J Am Med Assoc 1958; 166: 762-3.
14. Sacks AH. Theoretical prediction of a time-at-pressure curve for avoiding pressure sores. J Rehabil Res Dev 1989; 26(3): 27-34.
15. Bennett L, Lee BY. Vertical shear existence in animal pressure threshold experiments. Decubitus 1988; 1(1): 18-24.
16. Neumark OW. Deformation, not pressure, is the prime cause of pressure sores. Care Science and Practice 1981; 1: 41-6.
17. Hussain T. Experimental study of some pressure effects on tissue, with reference to bed-sore problem. J Pathology Bacteriology 1953; 66: 347-58.
18. Reswick JB, Rogers JE. Experiene at Rancho Los Amigos hospital with devices and techniques to prevent pressure sores. In: Kenedi RM, Cowden JM, Scales JT, editors. Bedsore Biomechanics. Baltimore: University Park Press, 1976; 301-10.
19. Allen V, Ryan DW, Lomax N, Murray A. Accuracy of interface pressure measurement systems. J Biomed Eng 1993; 15(4): 344-8.
20. Dabnichki P, Taktak D. Pressure variation under the ischial tuberosity during a push cycle. Med Eng Phys 1998; 20(4): 242-56.
21. Krouskop TA, Garber SL. Interface pressure confusion. Decubitus 1989; 2(3): 8.
22. Bader DL, Hawken MB. Pressure distribution under the ischium of normal subjects. J Biomed Eng 1986; 8(4): 353-7.
23. Bain DS, Scales JT, Nicholson GP. A new method of assessing the mechanical properties of patient support systems (PSS) using a phantom. A preliminary communication. Med Eng Phys 1999; 21(5): 293-301.
24. Conine TA, Daechsel D, Lau MS. The role of alternating air and Silicore overlays in preventing decubitus ulcers. Int J Rehabil Res 1990; 13(1): 57-65.
25. Vohra RK, McCollum CN. Pressure sores. BMJ 1994; 309(6958): 853-7.
26. Bliss MR. Pressure sores. Clinical trials best way of assessing different matresses. BMJ 1995; 310(6972): 126.
27. Bennett L, Lee BY. Pressure versus shear in pressure sore causation. In: Lee BY, editor. Chronic Ulcers of the Skin. New York: McGraw Hill, 1985; 39-56.
28. Clark M. Continuous interface pressure measurement using an electropneumatic sensor. Care Science and Practice 1987; 5: 5-7.
29. Rithalia SV, Gonsalkorale M. Assessment of alternating air mattresses using a time-based interface pressure threshold technique. J Rehabil Res Dev 1998; 35(2): 225-30.
30. Attard J, Rithalia SV, Kulkarni J. Pressure relief characteristics in alternating pressure air cushions. Prosthet Orthot Int 1997; 21(3): 229-33.
31. McLeod AG. Principles of alternating pressure surfaces. Adv Wound Care 1997; 10(7): 30-6.
32. Guyton AC. In: Textbook of Medical Physiology (8th ed). Philadelphia: WB Saunders, 1991; 170-83.
33. Lem FC, de Vries J. Transcutaneous oxygen measurement in stroke: circulatory disorder of the affected leg. Arch Phys Med Rehabil 1997; 78(9): 998-1002.
34. Wyss CR, Harrington RM, Burgess EM, Matsen FA. Transcutaneous oxygen tension as a predictor of success after an amputation. J Bone Joint Surg Am 1988; 70(2): 203-7.
35. Huch A, Huch R. Technical, physiological and clinical aspects of trancutaneous PO2 measurements. In: Taylor DEM, Whamond J, editors. Noninvasive Clinical Measurement. London: Pitman, 1977; 171-85.
36. Severinghaus JW, Stafford M, Bradley AF. tcPCO2 electrode design, calibration and temperature gradient problems. Acta Anaesthesiol Scand Suppl 1978; 68: 118-22.
37. Sanada H, Kanagawa K, Inagaki M, Imae J, Nishimura M, Yoshio K, Hiramatsu TA. A study on the prevention of pressure ulcers: the relationship between transcutaneous PO2 in the sacral region and predictive factors for pressure ulcer development. Wounds 1995; 7: 17-23.
38. Clark LC Jr. Monitor and control of blood and tissue oxygen tensions. Transactions of the American Society of Applied Physiology 1956; 2: 41-8.
39. Severinghaus JW, Bradley AF. Electrodes for blood PO2 and PCO2 determinations. J Applied Physiology 1958; 13: 515-20.
40. Beran AV, Tolle CD, Huxtable RF. Cutaneous blood flow and its relationship to transcutaneous O2/CO2 measurements. Crit Care Med 1981; 9(10): 736-41.
41. Rithalia SVS, Booth S. Factors influencing transcutaneous oxygen tension. Intensive Care World 1985; 2: 126-32.
42. McCollum PT, Spence VA, Walker WF. Oxygen inhalation induced changes in the skin as measured by transcutaneous oxymetry. Br J Surg 1986; 73(11): 882-5.
43. Rithalia SV, Heath GH, Gonsalkorale M. Assessment of alternating-pressure air mattresses using a time-based pressure threshold technique and continuous measurements of transcutaneous gases. J Tissue Viability 2000; 10(1): 13-20.
44. Rithalia SV, Gonsalkorale M. Quantification of pressure relief using interface pressure and tissue perfusion in alternating pressure air mattresses. Arch Phys Med Rehabil 2000; 81(10): 1364-9.
45. Linden M. Can blood flow in separate small tubes be quantitatively assessed by high-resolution laser Doppler imaging? Med Biol Eng Comput 1997; 35(6): 575-80.
46. Schubert V, Fagrell B. Local skin pressure and its effects on skin microcirculation as evaluated by laser-Doppler fluxmetry. Clin Physiol 1989; 9(6): 535-45.
47. Murray A, Marjanovic D. Optical assessment of recovery of tissue blood supply after removal of externally applied pressure. Med Biol Eng Comput 1997; 35(4): 425-7.
48. Scheuplein RJ, Blank IH. Permeability of the skin. Physiol Rev 1971; 51(4): 702-47.
49. Crewe R. The role of the occupational therapist in pressure sore prevention. In: Barbenel JC, Forbes CD, Lowe GDO, editors. Pressure sore.. London: Macmillan, 1983; 121-32.
50. Mahanty SD, Roemer RB. Thermal response of skin to the application of localized pressure. Arch Phys Med Rehabil 1980; 60: 584-90.
51. Brattgard SO, Severinsson K. Investigations of pressure, temperature and humidity in the sitting area in a wheelchair. In: Asmussen E, Jorgensen K, editors. Biomechanics VI-B. Baltimore: University Park Press, 1978; 270-3.
52. Pye G, Bowker P. Skin temperature as an indicator of stress in soft tissue. Eng Med 1976; 5(3): 58-60.
53. Smolander J, Holmer I. Individual response to physical work in the heat in relation to sweating and skin blood flow. Int Arch Occup Environ Health 1991; 63(3): 225-6.
54. Brown AM, Pearcy MJ. The effect of water content on the stiffness of seating foams. Prosthet Orthot Int 1986; 10(3): 149-52.
55. Daniel RK, Hall EJ, MacLeod MK. Pressure sores-a reappraisal. Ann Plast Surg 1979; 3(1): 53-63.
56. Waterlow J. Tissue viability. Calculating the risk. Nurs Times 1987; 83(39): 58-60.
57. Flanagan M. Choosing pressure sore risk assessment tools. Prof Nurse 1997; 12(6 Suppl): S3-7.
58. Le KM, Madsen BL, Barth PW, Ksander GA, Angell JB, Vistnes LM. An in-depth look at pressure sores using monolithic silicon pressure sensors. Plast Reconstr Surg 1984; 74(6): 745-56.
59. Sangeorzan BJ, Harrington RM, Wyss CR, Czerniecki JM, Matsen FA. Circulatory and mechanical response of skin to loading. J Orthop Res 1989; 7(3): 425-31.
60. Wagner DR, Jeter KF, Tintle T, Martin MS, Long JM. Bioelectrical impedance as a discriminator of pressure ulcer risk. Adv Wound Care 1996; 9(2): 30-7.
61. Inman C, Firth JR. Pressure sore prevalence in the community. Prof Nurse 1998; 13(8): 515-20.
62. Petersen NC. The development of pressure sores during hospitalisation. In: Kenedi RM, Cowden JM, Scales JT, editors. Bedsore Biomechanics. London: Macmillan Press, 1976; 219-24.
63. Dealy C. The size of the pressure sore problem in a teaching hospital. J Advanced Nursing 1991; 16: 633-70.
64. Berjian RA, Douglass HO, Holyoke ED, Goodwin PM, Priore RL. Skin pressure measurements on various mattress surfaces in cancer patients. Am J Phys Med 1983; 62(5): 217-26.
65. Stewart TP, McKay MG, Magnano S. Pressure relief characteristics of an alternating pressure system. Decubitus 1990; 3(4): 26-9.
66. Sideranko S, Quinn A, Burns K, Froman RD. Effects of position and mattress overlay on sacral and heel pressures in a clinical population. Res Nurs Health 1992; 15(4): 245-51.
67. Graebe RH. A proposed evaluation method for biosuspension devices using decubitus threshold pressure concept. 30 Oct - 2 Nov, 1997; Graebe RH. 54th Annual Session, Am Congress of Rehabilitation Medicine .
68. Rithalia SVS. Suitability of transcutaneous PO2 monitoring when comparing support surfaces [Letter]. CARE - Science and Practice 1989; 7: 24-5.
69. Grindley A, Acres J. Alternating pressure mattresses: comfort and quality of sleep. Br J Nurs 1996; 5(21): 1303-10.
70. Pring J, Millman P. Evaluating pressure-relieving mattresses. J Wound Care 1998; 7(4): 177-9.
71. Denne WA. An objective assessment of the sheepskins used for decubitus sore prophylaxis. Rheumatol Rehabil 1979; 18(1): 23-9.
72. Bliss MR, Thomas JM. An investigative approach. An overview of randomised controlled trials of alternating pressure supports. Prof Nurse 1993; 8(7): 437-44.