

|
Author(s)
Peter Vowden Consultant Vascular Surgeon
Kathryn Vowden Clinical Nurse Specialist and Lecturer
|
Contents
|
|
Published:
March
2001
Last updated: March 2001 Revision: 1.0 |
Keywords: Doppler assessment; venous ulcer; mixed ulcer; ankle brachial pressure index.
A Doppler assessment is not diagnostic of venous ulceration but may be of value in defining a safe level of compression bandaging.
Although helpful in defining when compression bandaging is contraindicated, an ABPI is meaningless when used in isolation.
The majority of patients diagnosed with so-called 'mixed ulcers' in fact have ulcers of venous aetiology and develop arterial insufficiency over time.
All patients with an ABPI of less than 0.8 should be referred for specialist assessment.
In those patients for whom high compression bandaging is contraindicated, reduced compression may be appropriate in selected cases with further arterial investigations if the ulcer fails to respond to treatment.
An ankle brachial pressure index (ABPI) of 0.8 is seen by some as a definitive decision-making number and it has almost become the 'Holy Grail' of leg ulcer assessment. However, its pivotal position is not based on hard evidence and the time has perhaps come to question our reliance on 0.8 and to look again at the concept of the mixed ulcer.
In the case of 0.8 a lot. An ankle brachial pressure index (ABPI) of 0.8 has become a pivotal figure in the management of leg ulceration, defining the cut off point for high compression bandaging and is frequently taken as indicating the presence of a so-called 'mixed ulcer'. The ABPI is derived from the ratio of arm systolic pressure, taken as the best non-invasive estimate of central systolic pressure, and the highest ankle systolic pressure, as measured in each of the three named vessels at the ankle, for each limb [1] [2]. Details of the method to be used are given in Box 1.
The use of hand-held continuous wave Doppler ultrasound equipment to measure systolic pressure and ABPI calculation is now considered a mandatory part of the assessment of leg ulcer patients [3] [4]. It is a misconception that an ABPI of > 0.8 is diagnostic of a venous ulcer as at present there is no diagnostic test for venous ulceration. Rather the diagnosis is one of exclusion based on the presence of venous disease and the absence of other aetiological factors. Even in expert hands a proportion of ulcers labelled as venous will have no detectable venous disease [5] [6]. Although an ABPI of 0.8 may be helpful in defining an arbitrary cut off point for the use of high compression bandaging, it is however meaningless when used to define an ulcer category. Early work with Doppler established that a 'normal' ABPI was usually greater than or equal to 1 and that an ABPI of < 0.92 indicated the presence of arterial disease [7]. In practice it is rare to find ulceration caused by arterial disease in a limb with an ABPI of > 0.5, although a low ABPI may reduce treatment options or delay healing in any leg wound or ulcer irrespective of its aetiology.
The importance of the interaction of venous and arterial disease in the ulcer process has been recognised for a number of years. Cornwall [8] [9] [10], reporting the Harrow experience, was among the first to suggest the use of Doppler ABPI measurement in the assessment of patients with leg ulceration. Both Creutzig et al [11] and Schultz-Ehrenburg [12] recognised the need for special guidelines for the management of mixed venous arterial ulcers. Cornwall et al [13] considered that an ulcer occurring in a limb with an ABPI of less than 0.9 should be considered ischaemic and that a pressure index below 0.75 had a significant impact on clinical management. This paper would appear to be the first reference linking ABPI to compression therapy. Callam et al [14][15] reported on the incidence of skin necrosis and amputation due to compression and recognised both the concept of 'mixed' ulceration (ulceration in a limb with both venous and arterial disease) and the need for reducing the compression levels in patients with an ABPI of 0.7 or less. The authors recognise however that this was a somewhat empirical approach which needed further study. The study, as far as the literature shows, has never been conducted. Blair et al [16] reported a large venous ulcer study using high compression therapy. In this seminal paper, patients were excluded from receiving high compression bandages when the ABPI was less than 0.8 as this group were felt to be at risk of necrosis from high compression bandaging. No rationale was given as to why 0.8 was used as a cut off point and yet an ABPI of 0.8 has become the accepted endpoint for high compression therapy, the trigger for referral for a vascular surgical opinion and the defining upper marker for an ulcer of mixed aetiology. This reliance on an ABPI of 0.8 was restated in the nursing literature by Cornwall [17] and has been stated on many occasions since.
Venous disease is common, and becomes more common with increasing age. It is therefore not surprising that venous leg ulceration may at times coexist with arterial disease. Nelzen et al [18] in a cross-sectional population study found that the ABPI was 0.9 or less in 185 (40%) of ulcerated legs. Venous insufficiency was the dominating causative factor in 250 legs (54%), of which 60% was the result of deep venous insufficiency. Arterial insufficiency was judged to be the possible dominating factor in 12% and 6% of limbs clearly showed ischaemic ulcers. Ghauri et al [19] found a 17% incidence of co-existing arterial and venous disease while Liew and Sinha [20] identified 13% and Scriven et al [21] 14% of patients with 'mixed' ulcers. Yet, is it correct to regard these patients as having mixed ulcers? The term implies that the ulcer has a dual aetiology. The work of Simon et al [22], however, would contradict this and suggests that over time a patient with a venous ulcer may have a slowly reducing ABPI. When reviewing patients with healed venous ulceration over a 12 month period they found that in 29% of patients, the ABPI fell over time and that seven patients (9%) developed arterial insufficiency as defined by an ABPI of less than 0.8. This drift towards arterial insufficiency over time is recognised in the recommendations to reassess patients receiving any form of compression at regular three monthly intervals, or earlier if symptoms change [3] [4]. Fowkes and Callam [23] in a study comparing leg ulcer patients with age and sex matched controls concluded that arterial disease was found no more frequently in venous ulcer patients than in controls, suggesting that arterial disease is not a risk factor for chronic leg ulceration.
It is likely therefore that the majority of patients diagnosed as having 'mixed ulcer' in fact have ulcers of venous aetiology, but that the use of high compression bandaging is contraindicated. Figure 1 illustrates the therapeutic continuum that should be considered when treating patients with vascular lower limb ulceration. Over time patients will progress down the slope as the ABPI falls with increasing age.
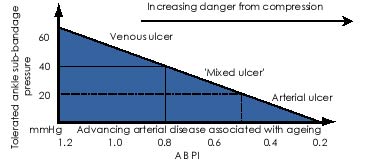
Clearly it is wrong to regard 0.8 as an absolute cut off point as it neither defines the transition between venous and arterial ulceration nor takes into account differences in perfusion pressure between the three vessels at the ankle - a pressure difference of 15 mmHg or greater indicates a proximal stenosis or occlusion in the vessel with the lower pressure [7]. Such a pressure difference will increase the risk of pressure damage to the related zone of the calf irrespective of the calculated ABPI for the limb. Recent work by Carser [24] also casts doubt on the reliance on a single value as a cut off point for treatment. This study demonstrates how variations in systolic pressure impact on the calculated ABPI, showing that patients with a low brachial systolic pressure have a higher mean ABPI and that reference to accepted criteria for high compression therapy in such a situation may lead to inappropriate compression and bandage damage.
Reliance on a single ratio also fails to take into consideration other factors that may be important when defining the level of compression to apply to any particular limb. These factors include: the limb shape; the presence of bony prominences; skin condition; the variability within the pressure measurement between the three ankle pulses; the presence of other diseases such as diabetes or rheumatoid arthritis; and the patient's tolerance of compression. In the group for whom high compression is considered inappropriate the treatment options are:
To correct the underlying arterial disease and then apply compression [19]
To use an alternative treatment such as intermittent pneumatic compression [26] [27] or alternative bandage systems such as short stretch [28].
UK national guidelines suggest that all patients with an ABPI below 0.8 should receive the benefit of specialist assessment [3] [4]. In our clinic, which acts as a tertiary referral centre, treatment is a joint decision between the vascular surgeon and the nurse specialist and is based on assessment and investigations. Arterial duplex ultrasonography provides a convenient non-invasive initial assessment method for these patients [25]. Figure 2 illustrates our management strategy.
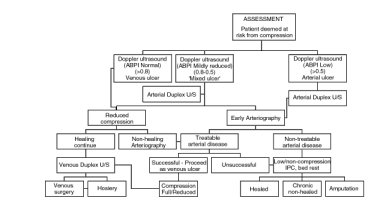
In patients with a reduced ABPI, a history of intolerance of compression or in whom there is a concern over the immediate introduction of high compression, such as in a diabetic patient with a marked peripheral neuropathy, we introduce three-layer compression omitting the third 3a bandage (Elset, Litepress or K-Lite) of the standard four-layer system. This method is favoured by Guest et al [25], preferring this to the 'slack' four-layer system suggested by Ghauri et al [19], which compromises on the durability of the bandage system. Short stretch bandages have been advocated in this situation [28] but our experience suggests that the majority of these patients are insufficiently mobile to benefit from this form of compression. If compression is not tolerated, or the ulcer shows little or no sign of healing within six weeks we would proceed to duplex ultrasonography and/or arteriography with a view to angioplasty or surgery if feasible.
In our experience the majority of patients with a so-called 'mixed ulcer' often present with a previous ulcer history extending back over several decades. In such patients we would introduce reduced compression resorting to further arterial investigations only if the ulcer failed to respond to this treatment, the ABPI continued to fall or is below 0.5 at presentation, or the patient's symptoms, such as claudication or rest pain, required intervention. Patients presenting with primary lower limb ulceration and an ABPI of 0.5 or less, even if they have varicose veins or deep venous insufficiency, should be treated from the onset as having arterial ulcers. In these patients venous disease, especially if associated with lipodermatosclerosis or atrophy blanche, should be treated as a secondary event once the arterial insufficiency is corrected.
There will remain a few cases which fall outside of these two groups. In these patients we generally use reduced compression as the primary treatment if the ABPI is above 0.5, but only after fully evaluating the patient and their limb perfusion. In some cases this will entail a duplex ultrasound arterial assessment or arteriography. Some patients do not respond to compression and will be unsuitable for arterial intervention. In this group we would advocate the use of alternative treatments such as bed rest or intermittent pneumatic compression with the device set to the individual's needs and tolerances.
Inappropriate use of high compression bandaging is dangerous and may place a limb at risk of damage and possibly even amputation. It is therefore important that these dangers are minimised. Doppler ABPI remains one of the cornerstones of the assessment process aimed at reducing bandage pressure damage, but it is only one element in the overall assessment of the patient and must not be used in isolation.
2. Stubbing NJ, Bailey P, Poole M. Protocol for accurate assessment of ABPI in patients with leg ulcers. J Wound Care 1997; 6(9): 417-8.
3. Royal College of Nursing. The Management of Patients with Venous Leg Ulcers. RCN Institute, 1998.
4. SIGN. The Care of Patients with Chronic Leg Ulcer. SIGN Secretariat, 1998.
5. Bjellerup M. Hydrostatic leg ulcers: a new classification. J Wound Care 1997; 6(9): 408-10.
6. Guest M, Smith JJ, Sira MS, Madden P, Greenhalgh RM, Davies AH. Venous ulcer healing by four-layer compression bandaging is not influenced by the pattern of venous incompetence. Br J Surg 1999; 86(11): 1437-40.
7. Sumner DS. Non-invasive assessment of peripheral arterial occlusive disease. In: Rutherford KS, editor. Vascular Surgery (3rd edition) Philadelphia: WB Saunders, 1998; 41-60.
8. Cornwall JV. Guidelines to leg ulcer care. Nursing (Lond) 1983; 2(11): 317-9.
9. Cornwall JV. Treating leg ulcers. J District Nursing 1985; 4(4): 4-6,11.
10. Cornwall JV. Diagnosis of leg ulcer. J District Nursing 1985; 4(3): 4-6.
11. Creutzig A, Wuppermann T, Hanauske U, Wrabetz W, Alexander K. [Oxygen pressure fields in ulcers of the lower leg]. Hautarzt 1985; 36(11): 612-6.
12. Schultz-Ehrenburg U. [Current therapeutic guidelines and differential diagnosis of venous leg ulcers]. Hautarzt 1985; 36(4): 212-7.
13. Cornwall JV, Dore CJ, Lewis JD. Leg ulcers: epidemiology and aetiology. Br J Surg 1986; 73(9): 693-6.
14. Callam MJ, Harper DR, Dale JJ, et al. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley leg ulcer study. Br Med J (Clin Res Ed) 1987; 294(6577): 929-31.
15. Callam MJ, Harper DR, Dale JJ, Ruckley CV, Prescott RJ. A controlled trial of weekly ultrasound therapy in chronic leg ulceration. Lancet 1987; 2(8552): 204-6.
16. Blair SD, Wright DD, Backhouse CM, Riddle E, McCollum CN. Sustained compression and healing of chronic venous ulcers. BMJ 1988; 297(6657): 1159-61.
17. Cornwall JV. Managing venous leg ulcers. Community Outlook 1991; May: 36-8.
18. Nelzen O, Bergqvist D, Lindhagen A. Leg ulcer etiology--a cross sectional population study. J Vasc Surg 1991; 14(4): 557-64.
19. Ghauri AS, Nyamekye I, Grabs AJ, Farndon JR, Poskitt KR. The diagnosis and management of mixed arterial/venous leg ulcers in community-based clinics. Eur J Vasc Endovasc Surg 1998; 16(4): 350-5.
20. Liew I, Sinha S. A leg ulcer clinic: audit of the first three years. J Wound Care 1998; 7(8): 405-7.
21. Scriven JM, Hartshorne T, Bell PR, Naylor AR, London NJ. Single-visit venous ulcer assessment clinic: the first year. Br J Surg 1997; 84(3): 334-6.
22. Simon DA, Freak L, Williams IM, et al. Progression of arterial disease in patients with healed venous ulcers. J Wound Care 1994; 3(4): 179-80.
23. Fowkes FGR, Callam MJ. Is arterial disease a risk factor for chronic leg ulceration?. Phlebology 1994; 9(2): 87-90.
24. Carser DG. Do we need to reappraise our method of interpreting the ankle brachial pressure index?. J Wound Care 2001; 10(3): 59-62.
25. Guest M, Williams A, Greenhalgh R, Davies A. Mixed leg ulcers. Eur J Vasc Endovasc Surg 1999; 18(6): 540-1.
26. Smith PC, Sarin S, Hasty J, Scurr JH. Sequential gradient pneumatic compression enhances venous ulcer healing: a randomized trial. Surgery 1990; 108(5): 871-5.
27. McCulloch JM, Marler KC, Neal MB, Phifer TJ. Intermittent pneumatic compression improves venous ulcer healing. Adv Wound Care 1994; 7(4): 22-4, 26.
28. Hofman D. Leg ulceration with mixed arterial and venous disease. J Wound Care 1997; 6(2): 53-5.
29. Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA 1969; 207(10): 1869-74.
30. Carter SA. Role of pressure measurement. In: Bernstein EF, editor. Vascular Diagnosis (4th edition) St Louis, Missouri: Mosby, 1993; 486-512.
31. Yao ST. Haemodynamic studies in peripheral arterial disease. Br J Surg 1970; 57(10): 761-6.
32. Yao JST. Pressure measurement in the extremity. In: Bernstein EF, editor. Vascular Diagnosis (4th edition) St Louis, Missouri: Mosby, 1993; 169-75.