Morphological Characteristics of the Dermal Papillae in the Development of Pressure Sores
Mechanisms of skin break down in the development of human pressure
sores are still unclear. This study was undertaken to clarify the
morphological characteristics of the dermal papillae in the skin
associated with pressure sores. Skin tissues were excised from the
sacrum of a Japanese subject post mortem, where a superficial pressure
sore had developed. Light microscopic and transmission and scanning
electron microscopic examinations were performed. It was found that
the atrophic, irregular contour and alignment of the dermal papillae
were characteristic of the boundary area between healthy and damaged
areas. In addition, a relatively dense network of collagen fibres in
the papillary layer of the boundary area was observed when compared
with the healthy area. These findings suggest that the morphological
changes of the papillae observed in the boundary area affect
micro-circulation, impairing tissue viability by inhibiting nutritive
blood supply and by accumulating metabolic by-products which
predispose to tissue damage.
Pressure sores are still a major problem affecting elderly and
bed-bound patients. Mechanisms of skin breakdown in the development
of human pressure sores are not fully understood. Most histological
studies on pressure sore development have been undertaken using animal
models
[1],
[2],
[3].
However, there is a limitation to the interpretation of
results obtained from animal experiments in terms of human pressure
sore development, since the structure of animal skin is not the same
as human's and animal models of pressure sores have been produced by a
single ischemic event using a mechanical indentor. We believe that human
pressure sores observed in the clinical area are a consequence of multiple
ischemic events produced by a combination of axial and shear stresses.
On the other hand, most human studies on pressure sore development
have been carried out by measuring physiological changes occurring in
the skin associated with pressure application, for example, skin blood
flow measurement
[4],
[5].
However, it is not sufficient to assume underlying
mechanisms of tissue break down in human pressure sores from results of
physiological data. Only a few studies are available for the understanding
of human pressure sores which use histological methods
[6],
[7].
Witkowski and
Parish
[6],
who attempted to investigate human pressure sore development
extensively, reported that the initial changes were found in the papillary
dermis where the capillaries and venules were greatly dilated showing
blanchable erythema. Barnett
[7],
developed a diagrammatic representation
of how a pressure sore develops in which the changes in collagen
characteristics in the papillary layer of the dermis were described.
The dermal papillae, which are the site providing oxygen and nutrition
to the epidermis, are of great importance in maintaining the integrity of
the skin and protecting the body from external forces. However, there is
little information available about morphological changes in the papillae
and how the collagen fibres beneath the papillae change during the course
of pressure sore development.
This study was undertaken to clarify the morphological characteristics
of the dermal papillae in the skin associated with pressure sores using
an electron microscopy.
Skin tissues containing healthy, boundary and damaged areas
associated with a pressure sore were excised from the sacrum of a Japanese
subject post mortem (87 years of age, female, death due to cerebral
infarction), 4 hours after death. The sore was identified as a stage
II pressure sore using the National Pressure Ulcer Advisory Panel's
classification
[8],
showing loss of epidermis and dermal papillae. The
skin tissues obtained were processed for the following examinations;
-
light microscopy,
-
transmission electron microscopy and
-
scanning electron
microscopy.
Light microscopy (LM).
Specimens were fixed in 10% formalin
and embedded in paraffin. Thick sections (5µm) were stained with
a silver impregnation procedure (Bielschowsky Gömöri method)
for identification of reticulin fibres and collagen fibres
[9],
[10].
Transmission electron microscopy (TEM).
Small tissue blocks were
fixed, both in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1M
cacodylate buffer, pH 7.4 (Karnovsky's fixative) and in cacodylate
buffered 2% osmium tetroxide 0.5% potassium ferrocyanide (pH 7.4). They
were dehydrated in a graded series of ethanol and embedded in epoxy
resin. Thin sections stained with uranyl acetate and lead citrate were
viewed using a transmission electron microscope (JEOL 100CX, JAPAN).
Scanning electron microscopy (SEM)
Karnovsky fixed tissue blocks
were immersed in 2N NaOH[11]
solution for 5 days at room temperature and
rinsed 4 times in physiological saline. NaOH maceration resulted in
removal of all cellular elements, but fibrous elements such as collagen
and reticulin fibres were left intact. The tissues were then postfixed
with cacodylate buffered 1% osmium tetroxide (pH 7.4) for two hours,
dehydrated in a graded series of ethanol, and dried by the t-butylalcohol
freeze drying method. The specimens were coated with gold, and viewed
using a scanning electron microscope (HITACHI S800, JAPAN).
Light micrographs of skin containing healthy, boundary and damaged
areas stained with silver are represented in
figure 1.
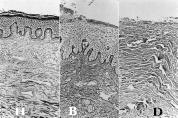 Figure 1:
Light micrographs of silver-impregnated sections from human skin.
Figure 1:
Light micrographs of silver-impregnated sections from human skin.
Both
the dermal papillae and papillary layer are considerably different in
structural appearance among the healthy (H), boundary (B) and
damaged (D) areas
(x 260)
In healthy tissue,
the papillary layer of the dermis in contact with the epidermis consisted
of relatively loose connective tissue in which collagen fibres were
scarce. In addition, it was noted that the most superficial surface of
this layer was bordered by a continuous thin sheet of reticulin fibres
(reticulin fibre sheet). In contrast, the reticular layer of the dermis
was composed of dense connective tissue containing collagen fibres
showing a feltwork appearance. The boundary between the healthy and
damaged areas was characterized by the presence of irregularly shaped
dermal papillae and a relatively dense network of collagen fibres in the
papillary layer. In the area damaged by a pressure sore, no epidermis
and dermal papillae were observed and the fibrous elements of the dermis
was exposed to the surface.
In combined SEM examinations with NaOH maceration, the epidermis and
epithelial basement membrane were effectively removed and numerous dermal
papillae were visualized three-dimensionally
(figure 2).
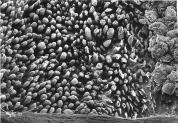 Figure 2:
SEM image of the outer surface of the dermis treated with NaOH.
Figure 2:
SEM image of the outer surface of the dermis treated with NaOH.
The epidermis is effectively removed and numerous dermal papillae with a
finger-like profile are visible in the healthy (H) and boundary (B)
area. No papillae are seen in the damaged (D) area.
(x 100)
The papillae in
the healthy region showed a finger-like configuration and were compactly
arranged
(figure 3H).
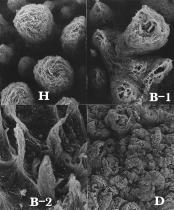 Figure 3:
Dermal papillae observed in the healthy (H), boundary (B) and damaged
area (D).
Figure 3:
Dermal papillae observed in the healthy (H), boundary (B) and damaged
area (D).
The papillae in the healthy area show a finger-like profile
and are regularly arranged.
In some papillae of the boundary area, the
top is broken (B-1) and the others show atrophic changes (B-2 ).
In
the damaged area an irregular contour with no papillae is shown
(H, B:
x 700, D: x 200)
In contrast, those in the boundary became atrophic
and were often broken having foraminae (figure 3B). No papillae existed
in the damaged area (figure 3D).
Higher magnification in SEM and TEM observations identified reticulin
fibres and collagen fibres. In the healthy area, the reticulin fibres
consisted of a delicate network of reticulin fibrils, 40-50 nm in diameter
which were distributed on the most superficial surface of the papillary
layer
(figure 4).
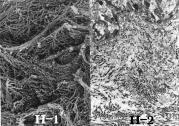 Figure 4:
High-power SEM (H-1) and TEM (H-2) images of the dermal papillae in
the healthy area.
Figure 4:
High-power SEM (H-1) and TEM (H-2) images of the dermal papillae in
the healthy area.
The surface of the papillary layer is covered
by a delicate network of reticulin fibrils, approximately 40 nm in diameter and
tiny spaces exist between reticulin fibrils
(H-1: x 20,600, H-2: x 14,300)
The reticulin fibrils were interwoven in slightly
loose networks with 30-60 nm sized spaces and formed a continuous sheet
(figure 4).
The collagen fibres consisted of bundles of collagen fibrils,
80-120 nm in diameter, which appeared to interlace in the papillary layer
of the dermis
(figure 5H).
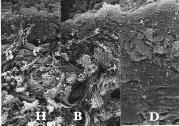 Figure 5:
Cut surface of the papillary layer.
Figure 5:
Cut surface of the papillary layer.
Bundles of the collagen fibrils (CB) are larger in size in the
boundary (B) than in the healthy (H) area.
In the damaged area (D), individual collagen fibrils cannot be
identified clearly
(x 3,300)
The collagen bundles in the papillary layer of
the healthy area were relatively loosely packed and were small in size,
approximately 0.6 to 1.8µm in diameter (figure
5H).
The reticulin fibre
sheet in the boundary was partially broken, having foraminae sized from
6µm to 20µm in diameter (figure 3B).
The collagen bundles distributed
beneath the reticulin fibre sheet became large in diameter and relatively
dense in the boundary when compared with the healthy
(figure 5B).
In the damaged area, the reticulin fibre sheet disappeared entirely
and the collagen bundles appeared to be largest and most densely packed
among three areas (figure 5D).
In addition, most of the collagen fibrils
in the damaged area were destroyed and cross-striated bands which are
normally seen in intact collagen fibrils were not observed
(figure 6.
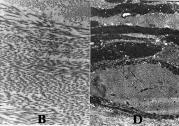 Figure 6: TEM images of collagen fibres in the papillary layer.
Figure 6: TEM images of collagen fibres in the papillary layer.
All collagen fibres are intact in the boundary (B),
but those in the damaged area (D) are
mostly degenerated
(B: x 9,300, D: x 8,400).
The present microscopic examinations clearly demonstrated the
morphological features of the papillary layer in the healthy, boundary
and ulcerated skin associated with the course of development of pressure
sores although this study was based on only one case. In the healthy area,
a finger-like configuration, high density and regular arrangement of
the dermal papillae were characteristic. Beneath the squamous epithelium
there was a dense network of reticulin fibres with small diameter (type
III collagen)[12],
maintaining spaces in between that allowed transport
of nutritional and tissue fluid. This space plays an important role in
maintaining viability of the tissue by exchanging metabolites.
However, at the boundary area, atrophy, an irregular shape and an
arrangement of the papillae containing foraminae were observed when
compared with the healthy area. Since the blood vessels, nerve fibres
and immunocompetent cells are situated beneath the reticulin fibre sheet
forming dermal papillae, it is assumed that these functions are also
affected. One of the roles of reticulin fibres is to fasten the basal
membrane of epidermis to the underlying dermis forming "rete pegs".
At
this interface, vital oxygen and nutrients diffuse into the basal layers
of the epidermis. Therefore, the rupture of this layer from whatever
cause, will have a serious effect on tissue viability resulting in
epidermal necrosis[7].
If tissue fluid flows into the interface through
discontinuities in the reticulin fibre sheet, nourishment for the
epidermis is inadequate, may be impaired, and the tissue fluid existing
in the area may contribute to the formation of a blister and subsequently
a break in the skin through which microorganisms can migrate.
In addition, reticulin fibres distributed in the papillary layer, became
fewer with increasing numbers of collagen fibres at the boundary. Stover
et al[13]
reported that type III collagen is less prevalent in the upper
dermis while type I collagen is more so in the reticular dermis in the
denervated skin of spinal cord injured patients who are known to be at
risk to pressure sore development. This finding is supported by a report
in which a decrease in type III collagen in the damaged upper dermis
associated with pressure sores was demonstrated using immuno-histochemical
analysis[14].
The atrophy and broken reticulin fibre sheet observed in the
boundary of this study may relate to the decrease in type III collagen in
the papillary layer. However it is not known how these changes in collagen
characteristics in the papillary layer of the dermis will affect the
biomechanics of the tissues and their viability during prolonged loading.
In the damaged area, there were no papillae and collagen fibrils
were destroyed. This indicates that in the damaged area, blood
supply and nourishment to maintain cell viability no longer function.
The morphological features of a pressure sore observed in this study,
in particular in the boundary area, correspond with the diagrammatic
representation of Barnett[7].
Further study is needed to confirm these results with a number of subjects
since the appearances of other ulcers might be different. Furthermore, in
order to establish an in-depth understanding of the aetiology of pressure
sore development, whether there is a correlation between physiological
changes measured at the skin surface and morphological changes observed
in the upper dermis of the skin, should be determined.
It was found that the atrophic, irregular shape and alignment
and presence of foraminae of the dermal papillae were characteristic
of the boundary area between healthy and ulcerated areas. These results
suggest that the morphological changes of the papillae and the papillary
layer observed in the boundary area may relate to impairment of tissue
viability in the development of pressure sores.
Satsue Hagisawa RN PhD,
Oita Medical University,
School of Nursing,
Hasama,
Oita 879-55,
JAPAN.
Tel: 81 97 586 5031
Fax: 81 97 586 5010
We are most grateful to H Kitamura and H Kawasato for
their technical supports in preparation of skin tissues and Profs Barbenel
and Ferguson-Pell, Bioengineering Unit, University of Strathclyde and
University College London, respectively, for their help in the preparation
of this paper.
-
1.
- Kosiak M. Etiology and pathology of
ischemic ulcers. Archives of Physical Medicine and Rehabilitation
1959; 40: 62-68.
-
2.
- Willms-Kretschmer K, Majno G. Ischemia of the
skin.
American Journal of Pathology 1969; 54: 327-343.
-
3.
- Daniel RK, Priest LD, Wheatley DC.
Etiologic factors in pressure sores: an experimental model.
Archives of Physical Medicine and Rehabilitation
1981; 62: 492-498.
-
4.
-
Hagisawa S, Barbenel JC, Kenedi RM.
Influence of age on postischemic reactive hyperemia.
Clinical Physics and Physiological Measure
1991; 123: 227-237.
-
5.
-
Schubert V, Fagrell B.
Evaluation of the dynamic
cutaneous postischemic hyperemia and thermal response in elderly
subjects and in an area at risk for pressure sores.
Clinical
Physiology 1991; 11: 169-182.
-
6.
-
Witkowski JA, Parish LC.
Histopathology of the decubitus ulcer.
Journal of the American Academy of Dermatology 1982; 6: 1014-1021.
-
7.
-
Barnett SE. Histology of the human pressure sore. CARE Science and Practice 1987; 5: 13-18.
-
8.
-
National Pressure Ulcer Advisory Panel.
Pressure ulcers incidence, economics and risk assessment.
CARE Science and Practice 1989; 7(4):
96-99.
-
9.
-
Gömöri G. Der mikrotechnische Nachweis unlöslicher
Kalksalze in den Geweben.
Virchows Archives 1934; 286: 682-689.
-
10.
-
Montes GS, Krisztan RM, Shigihara KM, Tokoro R, Mourao PAS, Junqueira
LCU.
Histochemical and morphological characterization of reticular fibers.
Histochemistry 1980; 65: 131-141.
-
11.
-
Ohtani O. Three dimensional organization of the connective tissue
fibers of the human pancreas: a scanning electron microscopic study of
NaOH treated tissues.
Archivum Histologicum Japonicum 1987; 50:
557-566.
-
12.
-
Fleischemajer R, Gay S, Meigel WN, Perlish JS.
Collagen in cellular and fibrotic stages of scleroderma.
Arthritis and Rheumatism
1978; 21: 418-428.
-
13.
-
Stover SL, Gay RE, Koopman W, Sahgal V, Gale LL.
Dermal fibrosis in spinal cord injury patients.
Arthritis and Rheumatism 1980; 23:
1312-1317.
-
14.
-
Yano H.
A study of mechanism of development of pressure sores and preventive
tools II.
Annual Report of Science and Technology Promotion Bureau, 1987 (Japanese).
This article was originally published in the Journal of Tissue
Viability 1998, Vol 8, No 3, pages 17-23.
The copyright of this article remains with the Tissue Viability Society.
All materials
copyright © 1992-Feb 2001 by SMTL, March 2001 et seq by SMTL
unless otherwise stated.
|
Home |
Index |
Subject Areas |
SMTL |
Site Map |
Archive |
Contact Us
|
http://www.worldwidewounds.com/1999/march/Hiromi-Arao/Dermal-Papillae.html
Last Modified: Thursday, 29-Mar-2001 14:27:01 BST


 Figure 1:
Light micrographs of silver-impregnated sections from human skin.
Figure 1:
Light micrographs of silver-impregnated sections from human skin.
 Figure 2:
SEM image of the outer surface of the dermis treated with NaOH.
Figure 2:
SEM image of the outer surface of the dermis treated with NaOH.
 Figure 3:
Dermal papillae observed in the healthy (H), boundary (B) and damaged
area (D).
Figure 3:
Dermal papillae observed in the healthy (H), boundary (B) and damaged
area (D).
 Figure 4:
High-power SEM (H-1) and TEM (H-2) images of the dermal papillae in
the healthy area.
Figure 4:
High-power SEM (H-1) and TEM (H-2) images of the dermal papillae in
the healthy area.
 Figure 5:
Cut surface of the papillary layer.
Figure 5:
Cut surface of the papillary layer.
 Figure 6: TEM images of collagen fibres in the papillary layer.
Figure 6: TEM images of collagen fibres in the papillary layer.