

| Dr Stephen Thomas Director Surgical Materials Testing Laboratory Bridgend and District NHS Trust Email: steve@smtl.co.uk |
Introduction
Test Materials Test Method Results Discussion. References |
| Bruce Fisher Quality Control Manager, SMTL Email: bruce@smtl.co.uk |
|
| Paul Fram Technical Officer, SMTL Email: paulf@smtl.co.uk |
Publishing Details Submitted: 31st March, 1998 Published: 7th April, 1998 Edition: 1.0 |
| Dr Mike Waring Product Development Manager ConvaTec Ltd Deeside Industrial Park |
The production of wound odour can represent a major problem for patients and their carers. If the formation of the odour cannot be prevented, it may be necessary to use a dressing that adsorbs the volatile molecules released from the wound which are responsible for the smell. The performance of four dressings each containing activated charcoal designed to be used in the treatment of malodorous wounds have been assessed in a laboratory-based study.
The results of this investigation suggest that differences exist in the performance of these products that may have potentially important clinical implications.
It is well recognised that certain types of wounds produce noxious odours which, even in moderate cases, can cause significant distress or embarrassment to a patient and their relatives. In extreme cases this odour can become so overpowering that it may cause an individual to withdraw from social contacts, even with family and close friends. [1] Despite these problems, odour absorbing dressings are currently not included in the list of reimbursable dressings in the United Kingdom and are therefore not available on prescription.
Wounds most commonly associated with odour production include leg ulcers and fungating (cancerous) lesions of all types. The smell from these wounds is caused by a cocktail of volatile agents that includes short chain organic acids, (n-butyric, n-valeric, n-caproic, n-haptanoic and n-caprylic) produced by anaerobic bacteria, [2] together with a mixture of amines and diamines such as cadaverine and putrescine that are produced by the metabolic processes of other proteolytic bacteria.
Organisms frequently isolated from malodorous wounds include anaerobes such as Bacteroides and Clostridium species, and numerous aerobic bacteria including Proteus, Klebsiella and Pseudomonas spp. Recent research has shown that the wound odour produced by some bacteria is specific to that species and that this may be analysed electrochemically to identify the presence of organisms such as beta-haemolytic streptococci. [3]
The most effective way of dealing with malodorous wounds is to prevent or eradicate the infection responsible for the odour. This may be achieved in a number of ways. The administration of systemic antibiotics or antimicrobial agents may be effective in some cases, but often the nature of the wound is such that it is not possible to achieve an effective concentration of the antibiotic at the site of infection by this method, particularly in the presence of slough or necrotic tissue. Most topical antiseptics are also likely to be of limited value and many of these have been shown to have adverse effects on wound healing. [4] [5]
One preparation that has been found to be effective in certain situations is a hydrogel containing a suitable concentration of metronidazole, typically about 0.8% w/v. Research has shown despite the fact that metronidazole is traditionally associated with the treatment of anaerobic infections, in the concentrations used topically it may also have an effect upon a range of aerobic organisms, [6] although the clinical evidence for the widespread use of this material has been questioned in the past. [7]
Less conventional methods of treating malodorous wounds include the the use of honey, [8] some varieties of which contain potent antimicrobial agents, and sugar, [9] either alone or in the form of a paste. The hyperosmotic environment produced by high concentrations of sugar is believed to inhibit bacterial growth, [10] and thus prevent odour formation. Live yoghurt is also sometimes applied in an attempt to encourage overgrowth of pathogenic organisms by lactic acid bacteria such as Lactobacillus bulgaricus and Streptococcus thermophilus.
More recently, larval therapy has been shown to be an extremely effective way of eliminating wound infection and odour from extensive necrotic wounds. [11] [12]
If for some reason, however, it is not possible to eliminate the bacteria responsible for the production of the odour it may be possible to deal with the problem by some other means. Historically wound odours were masked by burning incense and in more recent times, by the use of aerosols or air fresheners. Obviously although these do not resolve the underlying problem, they may make life a little more bearable for the patient and their family!
In 1976, a more scientific approach to the control of odour was reported by Butcher et al., [13] who described the use of a charcoal cloth developed by the Chemical Defence Establishment, Porton Down. This material was incorporated into pads containing surgical gauze and a layer of a water repellent fabric. When these pads were used in the treatment of fungating breast cancer, gangrene and immediate post operative colostomies, the associated odours were said to be totally suppressed.
Activated charcoal cloth is produced by carbonising a suitable cellulose fabric by heating it under carefully controlled conditions. During this process, the surface of the carbon breaks down to form small pores. These greatly increase the effective surface area of the fibres and hence their ability to remove unpleasant smells, as it is believed that the molecules that are responsible for the production of the odour are attracted to the surface of the carbon and are held there (adsorbed) by electrical forces. In the main these molecules are small and detected by the nose in low concentrations in the air. A single dressing which, by virtue of the large surface area of the carbon, is capable of taking up very large numbers of molecules should therefore prove capable of removing odour over prolonged periods.
Since 1976, a number of odour controlling dressings containing activated charcoal have been produced commercially. The first of these was Actisorb (Johnson & Johnson). In laboratory studies it was found that if the dressing was shaken with a suspension of bacteria, the organisms became firmly attached to the charcoal fabric and thus removed from the solution, although they still remained viable. A modified version of this dressing was therefore developed (Actisorb Plus) which contains 0.15% silver chemically bound onto the carbon. This imparts pronounced antibacterial activity to the dressing, killing the bacteria which are bound on to the cloth.
Since the introduction of Actisorb Plus, a number of dressings containing activated charcoal have been developed. Some of these, like Actisorb itself, are intended to be placed in direct contact with the wound. These products vary in structure and composition and hence in their ability to cope with wound exudate - often an important feature of malodorous wounds. Other products are designed as secondary dressings which are placed over a primary dressing, but beneath the retaining dressing or bandage. Examples include Clinisorb (Clinimed) and Denidor (Jeffreys, Miller & Co).
Despite the relatively widespread use of odour absorbing dressings, however, little objective comparative data is available on their odour and fluid handling characteristics. A number of possible test systems have been described for the odour absorbing properties [14] [15] [16] but two of these only considered the efficacy of the dressing in the dry state. The fluid handling properties of odour adsorbing dressings were considered in a separate study. [17] This may be important because the presence of liquids particularly those containing organic solutes, may have implications for the performance of the activated charcoal, competing for active sites with the molecules responsible for the odour and thus reducing its effectiveness.
Previous workers have used both chemical [14] [15] [18] and biological materials [16] [19] to test the efficacy of odour adsorbing dressings. The former technique is often favoured as the efficiency of the dressing can be determined using standard analytical techniques such as gas liquid chromatography. Determination of dressing performance using biological materials is currently restricted to more subjective methods of assessment such as the use of a human test panel. Lawrence et al, [19] adopted this second approach when they compared the odour absorbing properties of five dressings containing activated charcoal with that of a cotton gauze swab, acting as a control. A panel of volunteers were asked to assess the odour liberated from test samples after the addition of cultures of bacteria isolated from malodorous wounds. A similar approach was adopted by Griffiths et al., who also used microbiologically derived odours to test the performance of a range of products.
The aim of the current study was to develop a more objective test system that could be used to compare the ability of different dressings to prevent the passage of a volatile amine when applied to a wound model under simulated `in-use' conditions.
The products examined during the study are all intended to be used as primary wound dressings. They are;
 Figure 1 - Actisorb Plus
Figure 1 - Actisorb Plus
 Figure 2 - Actisorb Plus dressing cut open to
show structure.
Figure 2 - Actisorb Plus dressing cut open to
show structure.
 Figure 3 - CarboFlex
Figure 3 - CarboFlex
 Figure 4 - CarboFlex dressing cut open to
show structure.
Figure 4 - CarboFlex dressing cut open to
show structure.
 Figure 5 - Carbonet
Figure 5 - Carbonet
 Figure 6 - Carbonet dressing cut open to
show structure.
Figure 6 - Carbonet dressing cut open to
show structure.
 Figure 7 - Lyofoam C.
Figure 7 - Lyofoam C.
 Figure 8 - Lyofoam C cut open to show
structure.
Figure 8 - Lyofoam C cut open to show
structure.
 Figure 9 - Release
Figure 9 - Release
 Figure 10 - Release dressing cut open to show
structure.
Figure 10 - Release dressing cut open to show
structure.
This apparatus consists of a stainless steel plate bearing a central recess 50 mm diameter about 3 mm deep into which is inset a removable perforated stainless steel disc and a disposable Millipore pre-filter. Fitted to the stainless steel plate is an airtight perspex chamber with inlet and outlet ports.
 Figure 11 - Test apparatus showing dressing in
place.
Figure 11 - Test apparatus showing dressing in
place.
The test solution consists of sodium/calcium chloride containing 142 millimoles of sodium ions and 2.5 millimoles of calcium ions (values typical of those found in serum or wound exudate), to which is added 2% diethylamine and 10% newborn bovine serum (Flow Laboratories B/N 0030342).
This test solution is applied to the dressing through the perforated plate at a rate of 30ml/hour by means of a Vickers IP4 syringe pump (Vickers Medical, Sidcup). The concentration of diethylamine in the air in the perspex chamber is constantly monitored using a Miran 1B2 portable ambient analyser (Quantitech Ltd, Milton Keynes) and the measured values recorded electronically using a datalogger.
 Figure 12 - Complete testing rig.
Figure 12 - Complete testing rig.
The time taken for the concentration of diethylamine to increase by 10 ppm above baseline values was obtained from each dataset. From this value and the flow rate of syringe pump, the volume of fluid that had been applied to each dressing was calculated. The individual results for each sample of dressing together with the mean and standard deviation are shown in Table 1.
| Product | Test Volumes (ml) | Mean (s.d) | ||||
|---|---|---|---|---|---|---|
| Actisorb Plus | 8.9 | 11.0 | 10.8 | 9.0 | 7.8 | 9.5 (1.36) |
| Carboflex | 15.0 | 16.8 | 16.8 | 16.5 | 20.3 | 17.1 (1.95) |
| Carbonet | 10.2 | 13.1 | 17.5 | 15.3 | 11.4 | 13.5 (2.95) |
| Lyofoam C | 8.7 | 12.5 | 15.9 | 16.5 | 9.92 | 12.7 (3.48) |
| Release | 4.1 | 3.1 | 4.1 | 3.7 | 4.6 | 3.9 (0.53) |
To provide a visual comparison of the performance of all the different dressings, a composite figure was produced by selecting a single dataset from the results of each of the dressings examined (Figure 13). In order to ensure that these graphs were representative of the product concerned, the dataset that was chosen to construct each curve was that which most closely approximated to the mean value shown in Table 1
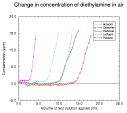 Figure 13 - Change in concentration of
diethylamine over time.
Figure 13 - Change in concentration of
diethylamine over time.
The results of this study demonstrate clear differences in the ability of the products to contain the test solution and prevent the loss of the volatile diethylamine into the surrounding air. Release, an absorbent dressing which contains no activated charcoal, is able to delay the passage of the diethylamine through the dressing but is less effective in this regard than Actisorb Plus which has limited absorbency. This suggests that the odour absorbing properties of the dressing are determined by at least two factors, the physical absorbency - a function of the presence of some form of absorbent layer, and the activity of the charcoal cloth itself. Products which combine a physical absorbent with a charcoal component show enhanced performance as might be anticipated.
What is not clear from the results, however, is whether the odour absorbing component of a dressing is likely to be most effective when applied directly to the surface of a wound or when it is incorporated into the structure of the pad or used as a secondary dressing. Further work is required to determine if the performance of the product is impaired once the dressing is saturated with wound fluid.
A previous study has shown that leg ulcers exudate at the rate of about 0.5 ml/cm2/24 hours. [21] A wound with an area of 10 cm2 could therefore be expected to produce about 5 ml of exudate in 24 hours. Based upon the results of the present study, therefore, the dressings examined could be expected to provide a degree of odour control varying from about 12 hours to three days. It is important to note, however, that the rate at which the test solution was applied during these tests was 30 ml per hour (36.7 ml/cm2/24 hours) and this value is considerably in excess of that encountered clinically. It could be argued, therefore, that this may have some effect upon the absolute performance of the dressings although it is considered unlikely that it would have a major effect upon the ranking order of the products as all these tests are comparative. Further investigations are planned to test the effect of flow rate on odour control.
A second possible criticism is the choice of diethylamine as the test material as this is not an agent normally found in wound exudate although it has a formula weight (FW) of 73, comparable with that of cadaverine, (H2N (CH2)5 NH2, (FW 102)) and putrescine, (H2N (CH2)4 NH2 (FW88)) molecules with which it shares some chemical similarities. Diethylamine has also been used on a number of occasions previously for assessing the performance of charcoal dressings. [14] [15] The use of an alternative test solution such as a bacterial culture was considered prior to commencing this work, but was discounted because no objective system was available to us that could be used to measure odour production from such a complex mixture.
Despite these potential criticisms, however, it is proposed that the technique described does provide, possibly for the first time, an objective method for comparing the performance of different odour absorbing dressings when challenged with a test solution containing an odiferous volatile amine under simulated conditions of use.