

| Dr S. Thomas, Director, P Loveless, Technical Manager, Surgical Materials Testing Laboratory, Bridgend and District NHS Trust, Bridgend, South Wales, CF31 1JP. Publishing details: Submitted: 23 June 1997 Published: 14 July 1997 Edition: 1.0 |
Table of Contents
Abstract Introduction Materials Methods Results Discussion Conclusions Acknowledgements References |
 Samples tested in the study.
Samples tested in the study.
In the presence of wound exudate the adhesive mass absorbs liquid and forms a gel, the properties of which are determined by the nature of the formulation. Some dressings form a cohesive gel, which is largely contained within the adhesive matrix; others form more mobile, less viscous gels which are not retained within the dressing structure.
In the intact state most hydrocolloid sheet dressings are impermeable to water vapour, but as the gelling process takes place, the dressing becomes progressively more permeable. The loss of water through the dressing in this way enhances the ability of the product to cope with exudate production.
Hydrocolloid sheets are widely used as primary dressings in the management of many different types of wounds including leg ulcers, burns, donor sites and pressure sores. With one or two exceptions, however, their relatively limited fluid handling properties tend to restrict their use to the management of light to moderately exuding wounds. For more heavily exuding wounds, products made from alginate fibre or polyurethane foam are often preferred.
Thinner versions of hydrocolloid dressings are also available and these may be used as alternatives to vapour permeable film dressings in the management of superficial injuries, or as secondary dressings over products such as alginates or hydrogels.
The majority of hydrocolloid dressings are broadly similar in appearance and it is therefore often assumed by purchasers and users alike that they will perform in a similar fashion. This study was carried out to compare the key properties of a number of dressings of European origin, using a series of different laboratory tests in order to test the validity of this assumption.
| Item | Size | Batch/Lot No | Origin |
|---|---|---|---|
| Tegasorb Thin (3M Health Care) | 10x10cm | MAR95007 | UK |
| Tegasorb (3M Health Care) | 10x10cm | 01794L01 | UK |
| Cutinova hydro (Beiersdorf AG) | 10x10cm | 50850021 | Germany |
| Askina Biofilm Transparent (Braun) | 10x10cm | 1730 | Germany |
| Askina Transorbent (Braun) | 10x10cm | C1795 | UK |
| Comfeel: Plus Plaques Biseautees (Coloplast) | 10x10cm | 47341.01 | France |
| Comfeel: Plus Transparenter (Coloplast) | 5x7cm | 47859.03 | France |
| Comfeel: Plus Flexibler (sample 1) (Coloplast) | 10x10cm | 48434.17 | France |
| Comfeel: Plus Flexibler (sample 2) (Coloplast) | 10x10cm | 48434.17 | France |
| Varihesive E (ConvaTec) | 10x10cm | 94119044 | Germany |
| Granuflex (ConvaTec) | 10x10cm | 94107772 | UK |
| Hydrocoll (Hartmann) | 10x10cm | 4390603 | Germany |
| Algoplaque(sample 1) (Laboratieres Urgo) | 10x10cm | 25 | France |
| Algoplaque(sample 2) (Laboratieres Urgo) | 10x10cm | 27 | France |
 Wallace Thickness Gauge.
Wallace Thickness Gauge.
 Paddington cup apparatus.
Paddington cup apparatus.
The cups were then securely sealed, weighed and placed in an inverted position in an incubator at 37°C together with a tray containing 1kg of freshly regenerated self indicating silica gel for a period of 24 hours.
At the end of this time the cups were removed from the incubator, allowed to equilibrate to room temperature and reweighed. From these results, the loss in weight of each cup due the the passage of moisture vapour through the dressing was determined by difference.
The base of each cup was then removed and any free fluid remaining in the cup that had not been absorbed by the dressing was allowed to drain away. The cup was then reweighed once again and the weight of fluid retained by the dressing calculated.
The test was then repeated twice more with incubation periods of 48 and 96 hours.
A sample of each dressing in turn was applied to a Paddington cup containing 20 ml of test solution. The cup was then placed in an inverted position upon the pan of a top pan balance in an incubator at 37°C. The balance was connected to an electronic data capture device which continually recorded changes in the weight of the cup resulting from the loss of moisture vapour through the dressing. A tray containing 1 kg of freshly dried silica gel was placed in the bottom of the incubator to maintain a low relative humidity within the chamber. After 48 hours the recorded data was down-loaded for examination.
This consists of a chamber, open at one end, bearing a flange with an internal diameter of 50 mm. A retaining ring with the same internal diameter as the hole in the flange is mounted over the open end of the cylinder which can be lowered down onto the flange by means of a screw thread. A sample of the dressing under examination is placed on the flange and held firmly in place by means of the retaining ring.
 Conformability testing apparatus.
Conformability testing apparatus.
Five samples of each dressing were tested in the way and the results are shown in Table 7
In this test the conformability of a dressing is considered to be inversely proportional to the pressure required to distort it by a predetermined amount.
After 2, 4, 24 and 48 hours, the bags were removed from the solution and after careful examination, gently blotted to remove excess liquid from the outer surface. They were then reweighed. From these weighings, the change in weight of each sample at each time interval was calculated by difference.
| Product | Thickness |
|---|---|
| (mm) (s.d) | |
| Comfeel + Transparenter | 0.37 (0.03) |
| Tegasorb Thin | 0.46 (0.02) |
| Askina Biofilm | 0.57 (0.02) |
| Algoplaque | 0.95 (0.02) |
| Hydrocoll | 1.09 (0.04) |
| Comfeel + Biseautees | 1.23 (0.02) |
| Tegasorb | 1.24 (0.03) |
| Comfeel + flexibler | 1.28 (0.02) |
| Cutinova hydro | 1.75 (0.03) |
| Varihesive E | 2.43 (0.03) |
| Granuflex | 2.44 (0.03) |
| Askina transorbent | 2.78 (0.04) |
These values are shown in Table 3, Table 4, and Table 5, and summarised graphically in Figure 1, Figure 2, and Figure 3.
| Dressing | Moisture Vapour Loss grams (s.d) |
Weight Absorbed grams (s.d) |
FHC grams (s.d) |
|---|---|---|---|
| Tegasorb Thin | 0.58 (0.03) | 2.89 (0.26) | 3.47 ( 0.26) |
| Tegasorb | 0.60 (0.22) | 4.40 (0.11) | 5.01 (0.26) |
| Cutinova Hydro | 0.67 (0.03) | 2.53 (0.05) | 3.21 (0.05) |
| Askina Biofilm Transparent | 0.27 (0.02) | 0.24 (0.07) | 0.51 (0.08) |
| Askina Transorbent | 3.35 (0.51) | 0.80 (0.05) | 4.16 (0.55) |
| Comfeel Plus Plaques Biseautees | 1.33 (0.05) | 1.53 (0.04) | 2.86 (0.06) |
| Comfeel Plus Transparenter | 0.65 (0.10) | 3.98 (0.12) | 4.63 (0.16) |
| Comfeel Plus Flexibler (sample 1) |
0.49 (0.05) | 3.27 (0.09) | 3.76 (0.11) |
| Comfeel Plus Flexibler (sample 2) |
0.77 (0.12) | 3.33 (0.06) | 4.10 (0.11) |
| Varihesive E | 0.03 (0.01) | 1.94 (0.11) | 1.97 (0.12) |
| Granuflex | 0.03 (0.02) | 1.75 (0.13) | 1.78 (0.14) |
| Hydrocoll | 0.50 (0.04) | 5.62 (0.11) | 6.12 (0.12) |
| Algoplaque | 0.22 (0.45) | 1.32 (0.14) | 1.54 (0.38) |
| Dressing | Moisture Vapour Loss grams (s.d) |
Weight Absorbed grams (s.d) |
FHC grams (s.d) |
|---|---|---|---|
| Tegasorb Thin | 1.46 (0.07) | 3.70 (0.07) | 5.17 (0.08) |
| Tegasorb | 2.27 (1.06) | 5.25 (0.39) | 7.52 (0.69) |
| Cutinova Hydro | 1.92 (0.02) | 2.87 (0.04) | 4.80 (0.06) |
| Askina Biofilm Transparent | 0.38 (0.02) | 0.12 (0.14) | 0.50 (0.13) |
| Askina Transorbent | 3.90 (0.38) | 0.75 (0.03) | 4.64 (0.38) |
| Comfeel Plus Plaques Biseautees | 2.28 (0.21) | 4.39 (0.05) | 6.67 (0.19) |
| Comfeel Plus Transparenter | 3.02 (0.21) | 1.68 (0.12) | 4.70 (0.28) |
| Comfeel Plus Flexibler (sample 1) | 1.55 (0.06) | 3.73 (0.09) | 5.28 (0.06) |
| Comfeel Plus Flexibler (sample 2) | 2.21 (0.12) | 3.45 (0.14) | 5.66 (0.16) |
| Varihesive E | 0.13 (0.03) | 2.77 (0.10) | 2.90 (0.11) |
| Granuflex | 0.10 (0.01) | 2.86 (0.08) | 2.96 (0.08) |
| Hydrocoll | 1.06 (0.05) | 6.46 (0.21) | 7.52 (0.25) |
| Algoplaque | 0.17 (0.03) | 2.81 (0.09) | 2.98 (0.07) |
| Dressing | Moisture Vapour Loss grams (s.d) |
Weight Absorbed grams (s.d) |
FHC grams (s.d) |
|---|---|---|---|
| Tegasorb Thin | 3.45 (0.11) | 3.59 (0.48) | 7.04 (0.51) |
| Tegasorb | 5.50 (0.75) | 5.57 (0.66) | 11.06 (0.19) |
| Cutinova Hydro | 4.04 (0.07) | 2.95 (0.05) | 6.99 (0.05) |
| Askina Biofilm Transparent | 0.79 (0.01) | 0.19 (0.16) | 0.98 (0.17) |
| Askina Transorbent | 9.89 (0.73) | 0.82 (0.05) | 10.72 (0.75) |
| Comfeel Plus Plaques Biseautees | 6.41 (0.31) | 4.45 (0.10) | 10.85 (0.31) |
| Comfeel Plus Transparenter | 9.31 (1.01) | 1.45 (0.22) | 10.77 (0.81) |
| Comfeel Plus Flexibler (sample 1) | 4.28 (0.75) | 4.03 (0.24) | 8.31 (0.77) |
| Comfeel Plus Flexibler (sample 2) | 5.67 (0.58) | 3.69 (0.14) | 9.36 (0.51) |
| Varihesive E | 0.91 (0.18) | 3.35 (0.11) | 4.25 (0.19) |
| Granuflex | 0.63 (0.47) | 3.35 (0.36) | 3.98 (0.13) |
| Hydrocoll | 2.19 (0.11) | 5.02 (0.69) | 7.19 (0.76) |
| Algoplaque | 0.44 (0.03) | 3.81 (0.19) | 4.25 (0.20) |
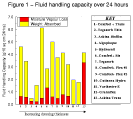 Figure 1 - Fluid
handling capacity over 24 hours. Figure 1 - Fluid
handling capacity over 24 hours. |
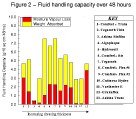 Figure 2 - Fluid
handling capacity over 48 hours. Figure 2 - Fluid
handling capacity over 48 hours. |
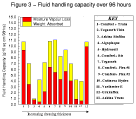 Figure 3 - Fluid
handling capacity over 96 hours. Figure 3 - Fluid
handling capacity over 96 hours. |
The values quoted in this table represent the total weight of fluid which passed through the dressing during this time. They do not, however, provide any information on the changes which occured in the permeability of each sample throughout this period. This information can be obtained by examination of the data recorded by the datalogger.
Typical moisture vapour transmission curves for each dressing have been combined together in Figure 4 to illustrate the differences between the different dressings examined.
| MVL (g/10cm2/48hrs) | ||||
|---|---|---|---|---|
| Dressing | Sample 1 | Sample 2 | Sample 3 | Mean |
| Tegasorb Thin | 1.38 | 1.66 | 1.56 | 1.53 |
| Tegasorb | 2.63 | 2.41 | 1.33 | 2.12 |
| Cutinova Hydro | 1.87 | 1.82 | 1.87 | 1.85 |
| Askina Biofilm Transparent | 0.51 | 0.50 | 0.53 | 0.51 |
| Askina Transorbent | 5.08 | 4.56 | 5.72 | 5.12 |
| Comfeel Plus Plaques Biseautees | 2.03 | 1.68 | 2.21 | 1.97 |
| Comfeel Plus Transparenter | 2.95 | 2.69 | 2.64 | 2.76 |
| Comfeel Plus Flexibler (sample 1) | 0.98 | 1.50 | 1.52 | 1.33 |
| Comfeel Plus Flexibler (sample 2) | 1.69 | 2.12 | 1.43 | 1.75 |
| Varihesive E | 0.20 | 0.15 | 0.17 | 0.17 |
| Granuflex | 0.17 | 0.17 | 0.12 | 0.15 |
| Hydrocoll | 1.02 | 1.08 | 1.05 | 1.05 |
| Algoplaque | 0.30 | 0.33 | 0.12 | 0.25 |
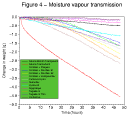 Figure 4 - Moisture
vapour transmission through hydrocolloid dressings
Figure 4 - Moisture
vapour transmission through hydrocolloid dressings
The conformability of the dressings as represented by the mean inflation pressure of the samples examined are summarised in Table 7 and expressed graphically in Figure 5.
| Dressing | Mean inflation pressure mmHg (s.d) |
|---|---|
| Tegasorb Thin | 107 (4.6) |
| Tegasorb | 187 (32.0) |
| Cutinova Hydro | 105 (3.7) |
| Askina Biofilm Transparent | 103 (5.7) |
| Askina Transorbent | [1] |
| Comfeel Plus Plaques Biseautees | 164 (11.8) |
| Comfeel Plus Transparenter | [2] |
| Comfeel Plus Flexibler (sample 1) | 166 (13.0) |
| Comfeel Plus Flexibler (sample 2) | 161 (8.9) |
| Varihesive E | 156 (4.6) |
| Granuflex | 162 (5.3) |
| Hydrocoll | 194 (20.4) |
| Algoplaque | 154 (17.3) |
| [1] Dressing is permeable to air and therefore
could not be tested. [2] Dimensions of dressing were too small for testing. |
|
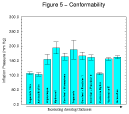 Figure 5 -
Conformability of hydrocolloid dressings
Figure 5 -
Conformability of hydrocolloid dressings
| Dressing | pH |
|---|---|
| Tegasorb Thin | 5.1 (0.04) |
| Tegasorb | 5.6 (0.02) |
| Cutinova Hydro | 5.6 (0.03) |
| Askina Biofilm Transparent | 6.0 (0.04) |
| Askina Transorbent | 7.0 (0.04) |
| Comfeel Plus Plaques Biseautees | 6.0 (0.07) |
| Comfeel Plus Transparenter | 6.4 (0.05) |
| Comfeel Plus Flexibler (sample 1) | 6.3 (0.11) |
| Comfeel Plus Flexibler (sample 2) | 6.6 (0.06) |
| Varihesive E | 4.5 (0.06) |
| Granuflex | 5.0 (0.01) |
| Hydrocoll | 5.5 (0.05) |
| Algoplaque | 5.8 (0.08) |
| Dressing | Fluid retention g/g of dressing after: | ||||
|---|---|---|---|---|---|
| 2 hours | 4 hours | 24 hours | 48 hours | 72 hours | |
| Tegasorb Thin | 2.15 (0.17) | 2.71 (0.20) | 4.63 (0.78) | 4.10 (0.61) | 3.79 (0.66) |
| Tegasorb | 1.09 (0.07) | 1.66 (0.12) | 4.06 (0.19) | 5.07 (0.19) | 5.31 (0.54) |
| Cutinova Hydro | 0.64 (0.010 | 0.89 (0.02) | 2.07 (0.04) | 2.85 (0.09) | 3.25 (0.06) |
| Askina Biofilm Transparent |
3.63 (0.08) | 3.96 (0.10) | 3.29 (0.83) | 1.29 (0.33) | 0.77 (0.21) |
| Askina Transorbent |
1.84 (0.23) | 2.49 (0.31) | 3.18 (0.53) | 2.77 (0.38) | 3.43 (0.27) |
| Comfeel Plus Plaques Biseautees |
1.38 (0.15) | 2.04 (0.17) | 4.56 (0.42) | 5.61 (0.24) | 5.85 (0.24) |
| Comfeel Plus Transparenter |
1.88 (0.35) | 2.14 (0.29) | 3.30 (0.23) | 3.54 (0.35) | 3.39 (0.31) |
| Comfeel Plus Flexibler (sample 1) |
1.01 (0.01) | 1.43 (0.03) | 3.54 (0.08) | 4.42 (0.26) | 5.24 (0.22) |
| Comfeel Plus Flexibler (sample 2) |
0.98 (0.05) | 1.42 (0.04) | 3.27 (0.29) | 4.03 (0.13) | 4.40 (0.24) |
| Varihesive E | 0.27 (0.05) | 0.40 (0.03) | 1.62 (0.08) | 2.03 (0.02) | 2.06 (0.08) |
| Granuflex | 0.27 (0.02) | 0.39 (0.02) | 1.53 (0.06) | 1.88 (0.02) | 1.50 (0.13) |
| Hydrocoll | 1.94 (0.04) | 2.35 (0.11) | 3.35 (0.15) | 2.52 (0.68) | 1.09 (0.54) |
| Algoplaque | 0.38 (0.07) | 0.46 (0.03) | 1.81 (0.05) | 2.06 (0.19) | 1.91 (0.10) |
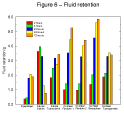 Figure 6 - Fluid
retention results 1. Figure 6 - Fluid
retention results 1. |
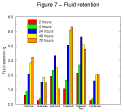 Figure 7 - Fluid
retention results 2. Figure 7 - Fluid
retention results 2. |
This clearly suggests that if fluid absorbtion and transmission by hydrocolloiod dressings were the only mechanisms involved in the control of exudate, many of the products examined in this study might be expected to perform poorly in the treatment of exuding wounds.
It has been demonstrated, however, that if a hydrocolloid dressing applied to a leg ulcer forms a secure waterproof seal onto the surrounding skin, it will actually reduce the amount of exdate produced by the wound by upto 50%.
It is believed that the chamber formed beneath the dressing becomes filled with exudate under pressure. As this pressure increases and approaches that within the capillaries, the loss of further fluid is inhibited. This effect will only occur, however, if the dressing forms an adequate seal over the wound. Once this seal is broken the mechanism fails.
The ability of some dressings to reduce exudate formation in this way may explain why some products which perform very poorly in laboratory studies appear to function satisfactorily in the clinical situation.
The results of the moisture vapour transmission test provide a further insight into the performance of the dressings and also highlight marked differences between the different brands. Examination of the moisture vapour transmission curves provides a useful indication of the rate at which the dressings absorb liquid, gel, and become permeable to water vapour.
The results of the conformability test suggest that there are potentially important differences between the products which may have some clinical relevance. It is likely that all of the dressings tested will become more conformable as they absorb exudate but a test conducted on a dry dressing provides an indication of its performance immediately after application. This may have implications for its ability to adhere satisfactorily to areas subject to movement such as ankles, elbows etc.
Extracts of the dressings were found to have a pH that ranged from 4.5 to 7 although the clinical significance of these observations is uncertain as little information is available upon the optimum pH required for wound healing.
The fluid retention/gel cohesion test represents a major challenge to the dressings. It is designed to determine how the products perform under more extreme conditions than they are likely to encounter in vivo. Nevertheless the results are not inconsistent with those of the more clinically relevant fluid handling capacity test.
Examination of the results of individual products reveals that in a few instances, after progressively increasing in weight with time as they absorb test solution, some samples appear to lose weight after 48 or 72 hours. This is because these dressings form soluble or mobile gels which can pass out through the fine nylon mesh in which the test samples are retained. It is of course possible that other dressings also lost some gel out of the nylon bags but that this effect was masked by the continued uptake of test solution.
In a clinical situation, those products which showed a significant decrease in weight in this test would probably leave residues of gel within the wound upon removal of the dressing. Conversely those products which did not demonstrate this effect will probably not leave significant residues upon removal but retain the gel within the structure of the dressing.
The introduction of a classification or grading system for hydrocolloid dressings based upon their ability to cope with fluid production would provide potential users with useful information to facilitate the selection process. Such a classification system should also take account of the conformability and ease of use of the products concerned.
The adoption of standard test methods for assessing the performance of hydrocolloid dressings would greatly facilitate this process. It is proposed that the methods used in the present study could form the basis of such a battery of tests.