

|
Author(s)
James Wright Staff Physician
|
Contents
|
|
Published:
May 2001
Last updated: May 2001 Revision: 1.0 |
Keywords: hyperbaric oxygen; wound healing; oxygen effects; infection.
Oxygen used under pressure or hyperbaric oxygen (HBO2) can assist wound healing.
HBO2 is considered unnecessary in simple, well-perfused wounds, but can be used successfully in hypoxic or ischaemic wounds such as diabetic wounds, venous stasis ulcers, failing grafts and flaps, necrotising soft tissue infections and refractory osteomyelitis.
In wound healing, hypoxia is an insufficient supply of oxygen which prevents normal healing processes. HBO2 provides the oxygen needed to stimulate and support wound healing.
HBO2 is able to combat clinical infection such as gas gangrene by acting directly on anaerobic bacteria, enhancing leukocyte and macrophage activity and potentiating the effects of antibiotics.
HBO2 is a relatively safe non-invasive therapy. Side effects include middle ear and pulmonary barotraumas and myopia. Contraindications include poor cardiac output and severe obstructive pulmonary disease.
Oxygen is one of the most versatile and powerful agents available to the modern medical practitioner. The therapeutic use of oxygen under pressure is known as hyperbaric oxygen therapy (HBO2) and has been used to assist wound healing for almost 40 years. HBO2 has several specific biological actions which can enhance wound healing processes. Hyper-oxygenation of tissue, vasoconstriction, down regulation of inflammatory cytokines, up-regulation of growth factors, antibacterial effects, potentiation of antibiotics, and leukocyte effects of HBO2 are discussed in relation to wound healing problems. This article looks at how a greater understanding of the biological and physiological effects of using oxygen under pressure can benefit patients with problem wounds.
HBO2 was first used to recompress divers by Behnke in the 1930s [1], and was developed to complement the effects of radiation in cancer treatment by Churchill-Davidson in the 1950s [2]. Within a few years HBO2 was being used to support patients undergoing cardiac surgery [3], and to treat clostridial gas gangrene [4] and carbon monoxide poisoning [5]. HBO2 was first used to assist wound healing when it was noted in 1965 that burns of the victims of a coal mine explosion, treated with HBO2 for their CO poisoning, healed faster [6]. In spite of this long history of therapeutic use, the mechanisms of action of HBO2 are still being discovered. As we learn more about how oxygen interacts with living organisms, new treatments and parameters of use are suggested. Today, the medical use of oxygen under pressure, or hyperbaric oxygen, is an evolving specialty.
Hypoxia can be defined as an insufficient supply of oxygen to support biological processes. It is possible to have hypoxia in one area of a wound and not in an adjacent area. Similarly hypoxia can be time-dependent with sufficient oxygen to support basal tissue maintenance at one time, but not enough to allow for growth or healing at another. Thus it is difficult to place an absolute number for PO2, which can be used to define hypoxia in all situations. Hypoxia in anaesthesia is defined as an oxygen saturation less than 90% or a PaO2 of < 60 mmHg [7]. This is clearly a higher level than the tissue oxygen pressure of 40 mmHg needed to reliably heal a leg wound [8][9]. In wound healing, hypoxia can be defined as an insufficient supply of oxygen to allow the healing process to proceed at a normal rate.
Not all effects of hypoxia are bad. In fact all wounds initially have areas of hypoxic tissue. Local hypoxia in the micro-environment of the wound causes several wound healing processes to occur such as leukocyte adherence, neovascularisation, collagen formation and bone formation. When hypoxia is severe, prolonged or widespread deleterious tissue effects can occur.
Ischaemia-reperfusion injury: When hypoxia extends beyond the local wound environment the effects of ischaemia-reperfusion injury can be seen. Reactive oxygen species are produced, including oxygen free radicals. Initially, these usually cause vasoconstriction followed by vasodilation, although the effects are dependent on vascular epithelial receptors in the tissue involved. Endothelial cell damage and release of prostaglandins, inflammatory cytokines (TNF-alpha and IL-6) and nitric oxide (NO) from the vascular endothelium occurs. Subsequent membrane peroxidation further increases the cellular damage.
As capillaries become leaky and interstitial oedema accumulates, circulation is further compromised with compounded injury. The surgical or medical re-establishment of interrupted circulation sends blood to the ischaemic area, providing new oxygen substrate for the formation of more free radicals, with the result that the injury temporarily worsens. In massive injury the release of inflammatory cytokines (and possibly free radicals) escapes the normal regulatory mechanisms and can lead to multiple organ failure. Hence a long and catastrophic chain of events can be initiated by oxygen deprivation.
HBO2 can provide pharmacological doses of oxygen to healing tissue. A typical wound care treatment dive consists of providing 90 minutes of 100% oxygen at 45 feet of sea water (fsw) - 13.7 m of sea water (msw) or 1.38 Bar. This is the equivalent of 2.36 (ATA) atmospheres of 100% oxygen. In recompression therapy for diving-related injuries a patient might be exposed to a minimum of four hours oxygen at depths varying from 60 fsw (18.3 meters, 2.8 atmospheres, 284 kilopascals) to the surface.
Hyperbaric chambers are either multiplace or monoplace. A multiplace chamber is able to treat several patients at one time with a medical attendant in the chamber Figure 1. The patients breathe oxygen through a mask or hood Figure 2. In a monoplace chamber one patient is treated in a small hyperbaric chamber filled with 100% oxygen Figure 3.


In a typical wound care treatment dive hyperbaric oxygen is capable of providing tissue oxygen levels of greater than 11 times normal, or up to 620 mmHg. Most chronic wounds are hypoxic and HBO2 provides the oxygen needed to stimulate and support wound healing. Some examples of typical levels of oxygenation provided in a hyperbaric oxygen treatment are illustrated in Table 1
| PO2 values | |||
| ATAs | 1.0 Air | 1.0 O2 | 2.4 O2 |
| air | 159 | 760 | 1824 |
| alveolar | 104 | 673 | 1737 |
| arterial | 100 | 660 | 1700 |
| venous | 36 | 60 | 1650 |
| muscle | 29 | 59 | 250 |
| subcutaneous | 40 | 200-300 | 250-500 |
| chronic wound | 15 | 200-400 | 660 |
| chest tcp02 | 67 | 450 | 1312 |
| foot tcp02 | 63 | 280 | 919 |
When used in wound healing HBO2 provides a short pulse of oxygen - 90 minutes in a 24-hour day. Although, such a short time provision of elevated oxygen could not significantly effect wound healing, HBO2 acts in numerous ways that affect the wound after the treatment has stopped. There are eight principal methods in which HBO2 is capable of affecting tissue:
Pressure effects of oxygen
Vasoconstrictive effects of oxygen
100% oxygen concentration effects on the diffusion gradient
Hyperoxygenation of ischaemic tissue
Down regulation of inflammatory cytokines
Up-regulation of growth factors
Leukocyte effects
Antibacterial effects.
Pressure effects of oxygen: The effect of elevating the ambient partial pressure of the inspired gas, usually to 2.38 ATAs, is unimportant in wound healing, but quite significant when dealing with the gas bubble diseases, decompression sickness and air gas embolism. At elevated pressures the harmful effects of gas bubbles in the tissue are minimised. Our research and that of others on wounds exposed to elevated pressures has demonstrated no evidence of an isolated pressure effect [10].
Vasoconstrictive effects of oxygen: The vasoconstrictive effects of HBO2 can be used to good effect to treat patients. HBO2 causes a significant reduction of oedema, which has been shown to be beneficial in reperfusion injury, crush injury, compartment syndrome, burns, wound healing, and failing flaps [11][12][13][14].
Oxygen diffusion effects: The diffusion of nitrogen out of the tissues in decompression sickness is facilitated by the use of 100% oxygen. In wound healing the beneficial effects of oxygen are primarily related to the concentration of oxygen molecules in the tissue, rather than by diffusion kinetics. However, the rate of oxygen entry into the wound environment is affected by the rate of diffusion from the capillaries. Oedema adversely affects the achievement of high oxygen concentrations in the wound and increases the intercapillary diffusion distance. Even a small increase in tissue oedema can dramatically slow the rate of entry of oxygen into the tissues and can cause tissue hypoxia.
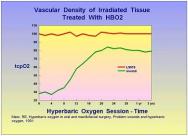
Hyperoxygenation of tissue: The oxygenation of hypoxic tissue is one of the key mechanisms by which HBO2 accelerates wound healing. Numerous studies have shown a dose response curve for the provision of oxygen in the wound healing environment [10][14][15][16][17][18][19]. However, oxygen is a powerful drug and just like other drugs, it is possible to give too little or too much. Chronic wounds are frequently hypoxic and the provision of HBO2 corrects the hypoxia, albeit temporarily. It then allows for acceleration of the wound healing process through processes which continue long after the HBO2 session has ended and tissue oxygen levels have returned to pre-treatment values. Over time the oxygenation of the chronic wound improves with HBO2 therapy. Marx and Johnson demonstrated that for irradiated wounds, HBO2 induces neovascularisation, which becomes significant after about 14 treatments and continues for years after the HBO2 therapy has ceased [20] Figure 4. A typical chronic wound will usually require 20 to 30 HBO2 treatments. This probably represents the amount of neovascularisation needed to sustain wound healing.
Cytokine down regulation and growth factor up-regulation: HBO2 is capable of favourably influencing a number of cytokines and growth factors important to wound healing. When administered after wounding, HBO2 up-regulates collagen synthesis through pro-al(I) mRNA expression [21]. In rabbit ear wounds HBO2 has been shown to up-regulate mRNA for the platelet-derived growth factor (PDGF)-beta receptor [22]. In ischaemic flaps HBO2 up-regulates fibroblast growth factor (FGF) causing an increased effect over that seen with FGF alone [23]. In situations where FGF is ineffective, HBO2 can render it highly effective [24], although the effect is different from up-regulation. In patients with Crohn's disease interleukin-1 (IL-1), IL-6, and tumour necrosis factor (TNF)-