

|
Author(s)
Stephen Thomas
Paul Fram
Pete Phillips
|
Contents
|
|
Published:
November 2007
Last updated: November 2007 Revision: 1.0 |
Keywords: wound exudate; wound dressings; exudate uptake; compression; WRAP project.
Excessive exudate production can have serious consequences for a wound and the surrounding skin.
Traditional cotton wool and lint dressings have a high fluid handling capacity but tend to shed fibres. In recent years foam dressings have been developed to overcome this problem.
Foam dressings must be able to perform optimally under compression when used in the treatment of venous leg ulcers.
A laboratory study compared the effects of compression on two widely used foam products to illustrate the importance of this parameter when assessing dressing performance.
The effective management of exudate is one of the principal requirements of any dressing system. Products used for the treatment of venous leg ulcers in particular should be capable of retaining significant volumes of fluid while subjected to clinically relevant levels of compression.
This paper describes one way in which such dressings can be evaluated in the laboratory, and provides anonymised data to illustrate the differences in performance between two widely used foam products when tested in this way.
The modern wound care practitioner has available to them a panoply of dressings designed to facilitate wound healing or improve the quality of life of patients with intractable non-healing wounds.
Dependent on their composition and structure, such dressings may be variously used to absorb exudate, combat odour and infection, relieve pain, promote autolytic debridement (wound cleansing) or provide and maintain a moist environment at the wound surface to facilitate the production of granulation tissue and the process of epithelialisation. Of all these functions, exudate management is generally regarded as the most important.
Wound exudate is a generic term used to describe the liquid produced from chronic wounds, fistulae or acute wounds once haemostasis has been achieved. Exudate is normally a pale straw-yellow colour but it can become discoloured by the presence of faecal matter or infection.
For centuries, the production of exudate was regarded as an inevitable consequence of the formation of many types of wounds. In more recent times, however, it has been recognised that exudate contains a complex mixture of bioactive molecules that have important effects (both positive and negative) on the healing process [1].
Because excessive exudate can cause maceration of the peri-wound skin, which in turn can lead to infection, considerable attention has been given by the industry to the development of dressings that prevent the accumulation of large volumes of fluid within a wound and prevent it from spreading over the surrounding healthy tissue. The ability of a dressing to deal with exudate is known as its fluid handling capacity.
Some dressings, such as hydrophilic polyurethane films, are very permeable to water vapour and thus permit the passage of a significant quantity of the aqueous component of exudate from the wound to the environment by evaporation.
In practice, however, most permeable products are unable to cope with the volume of fluid that is commonly produced by some types of wounds, and therefore the control of exudate by permeability to moisture vapour alone is generally insufficient to meet the clinical requirements of a dressing designed for the treatment of heavily exuding leg ulcers, burns or malignant wounds, for example.
In such situations, products that have the ability to absorb or otherwise retain significant quantities of liquid are required, although many also combine this absorptive function with a degree of permeability to maximise their total fluid handling potential.
To 'absorb' literally means to 'take in', 'suck up' or 'incorporate as part of itself'. In a wound management context this may involve the passage of liquid into spaces formed within the structure of the product, such as gaps between fibres or pores within foams, and/or the uptake of liquid by individual fibres from which the dressing is constructed.
The simplest and most familiar examples of surgical absorbents are absorbent cotton (cotton wool), lint and cotton gauze, all of which have been used for over a century to absorb blood and tissue fluid during and after surgery, either alone or in combination. However, fibre loss from these products into the wound can impair healing and result in the production of granulomas [2].
Foam dressings represented a significant advance over simple cellulose-based materials, by eliminating the potential problems of fibre loss. Early foam dressings had no purpose-designed wound contact layer and consisted of a piece of open cell foam into which granulation tissue could easily grow and which exudate and cellular debris could penetrate. As a result, the dressing effectively became part of a scab covering the wound, which was ripped off during dressing changes, causing additional trauma.
The importance of the ability of a dressing to perform adequately when partially compressed cannot be over-emphasised. A key part of the management of venous leg ulcers, for example, is the application of significant levels of compression, often as high as 40mmHg. Similarly, in the management of pressure ulcers, dressings are frequently required to function adequately when at least some part of the patient's body weight is bearing down upon them.
Simple foams, like bath sponges, initially take up large volumes of fluid, but a large amount of the retained liquid is lost if the sponge is gently compressed. If allowed to stand in one position, fluid will drain away under the effect of gravity.
Much research has therefore been devoted to maximising the performance of foam dressings by casting the foam from hydrophilic polymers and/or the inclusion of super-absorbents within the porous structure of the foam itself. These developments have resulted in the formation of a family of products that are among the most absorbent and widely used dressings available.
A fundamental requirement for anyone involved in developing new or improved absorbent dressings or any organisation that performs in vitro testing to obtain comparative results to facilitate dressing selection is a suitable test system that mimics, as closely as possible, the conditions that are encountered in normal clinical practice.
Key design criteria for a new wound model have been described in an earlier publication, as follows [3]:
Fluid should be provided to the test sample by some form of pump or other suitable positive flow device, which more closely simulates the clinical situation. A passive uptake technique is not acceptable
The fluid should not be presented to the test sample under excessive pressure. Previous test systems have, in effect, injected fluid into the dressing under pressure, although there is no evidence that this occurs in wounds
There must be some suitable method for controlling the temperature of the system for the duration of the test, to reproduce the environmental conditions in the wound
The test should provide some indication of the dynamic performance of the dressing, measuring its fluid handling capacity profile over time, and not just a single total absorbency figure
The equipment should be capable of delivering test solution at a range of different flow rates, so that the effect of different rates of exudate can be examined
The apparatus should indicate when either vertical or lateral strike-through has occurred
The apparatus should be suitable for testing a wide range of different types of dressings to permit direct comparison of the results. Previous methods were frequently dedicated to one type of technology, such as alginates
The equipment should be compatible with a range of different test solutions
Where appropriate, the apparatus should permit the application of varying loads to the test samples in order to determine the effect of external pressure
The apparatus should permit the measurement of moisture vapour transmission by the dressing as an integral part of the test
The test should be easy to perform and provide results that can be reproduced within and between laboratories
The equipment should not be excessively expensive to produce
The test should, ideally, provide an indication of the wear time of a dressing in normal clinical use.
The development of a prototype test rig (see Figure 1 and Figure 2) that was believed to meet most, if not all, of these requirements, was similarly described in the earlier publication [3]. A new version of this rig has been developed over recent years through collaboration with academia and industry through the Woundcare Research for Appropriate Products (WRAP) project (details available at: www.kcl.ac.uk/wrap).


Essentially it consists of a two-part stainless steel plate (the 'wound bed'), mounted on a Perspex table, with an electronically controlled heating mat that enables the test plate and sample under examination to be maintained within a narrow temperature band. The central section contains a recess into which a 15mm-long channel has been milled, joining a 3mm inlet hole with a 7mm outlet hole. The centre section is milled from block stainless steel, and includes two ports that permit the introduction and unimpaired exit of the test solution. The diameter and depth of the recess is sufficient to accommodate two absorbent pads that ensure the effective transfer of liquid from the channel to the dressing above.
Test fluid, fed through one of the ports by means of a syringe pump, travels along the narrow channel and out through the second port, falling vertically down through a short, wide-bore tube. This tube discharges into a receiver placed on the pan of an electronic balance. The liquid in the receiver is covered with a layer of oil to prevent loss by evaporation. The balance is connected to an electronic data capture device that records changes in the balance reading at predetermined intervals throughout the period of the test.
The test fluid usually used for absorbency testing consists of a solution of sodium/calcium chloride with an ionic composition similar to that of serum, although others are possible.
The new test rig is currently being evaluated by a multidisciplinary group comprising representatives from most European dressings companies, in addition to others with an interest in dressing design or performance. This group, which was originally funded by a research grant from the Engineering and Physical Sciences Research Council (EPSRC), is concerned with the development and validation of methodologies for medical device evaluation [4].
A dressing sample typically measuring 10cm x 10cm (total area of 100cm2) is secured on to the 'wound bed'. During use, as test fluid is applied to the test rig and passes along the open channel, some will be taken up by the dressing. Any unabsorbed fluid will continue to pass along the channel until it falls through the outflow pipe into the receiver, causing a change in the balance reading. The amount of fluid that accumulates in this way is inversely proportional to the absorbency of the dressing. A highly absorbent dressing may take up all the liquid that is applied to it, while less absorbent products will absorb only for a short time or take a little while to reach maximum absorbency.
During a test, therefore, the maximum weight of fluid that can be taken up by a dressing is determined by the flow rate of the syringe pump.
When performing laboratory-based tests to assess or compare the performance of medical devices, it is clearly important to ensure that the test conditions employed are as clinically relevant as possible. In the context of this investigation, this means ensuring that the flow rate of the simulated wound exudate is comparable with the volume of exudate produced by a heavily exuding wound. According to the literature, this is typically around 5ml per 10cm2 per 24 hours [5], but in the presence of infection this value can easily double [6].
For this reason, the syringe pump is normally set to deliver a nominal 1ml per hour as this value provides a reasonable compromise between clinical relevance and a need to keep testing times to a minimum for practical reasons.
In order to simulate levels of compression encountered clinically, each sample is typically loaded with a total mass of 5kg distributed over 100cm2, equivalent to 36.8mmHg. This pressure is chosen as it approximates to the maximum value that is usually used therapeutically.
In the treatment of leg ulcers caused by venous insufficiency, 40mmHg is widely quoted in the literature as the target pressure for compression bandaging systems such as the Charing Cross four-layer system first described in the late 1980s [7].
The aim of the present study was to use this equipment to compare the performance of two commonly used foam dressings (identified as Products A and B) in order to demonstrate the important effects of externally applied pressure on dressing performance.
For the purposes of this investigation, each dressing was tested in the compressed and uncompressed state, to determine whether they performed differently under compression.
The products were tested in duplicate, giving a total of eight runs (two dressings, in duplicate, with and without compression) and each test lasted approximately 20 hours.
During the course of the test, both dressings (Product A and Product B) absorbed all of the fluid supplied to them in the uncompressed state. In Figure 3 the plot contains eight different data sets. However, only three are visible due to the superimposition of some data sets on top of others. These data sets are superimposed on each other due to the fact that the absorption rate of each dressing was greater than the fluid delivery rate, and therefore the absorption rate equals the flow rate of the pump, giving a straight line.
In the presence of compression, however, although Product B was still able to absorb the total volume of fluid applied (straight line in Figure 3), the absorbency of Product A was considerably reduced, capable of absorbing only about 25% of the volume of fluid taken up by the comparator product under the same conditions (the two lower lines, orange and green, in Figure 3).
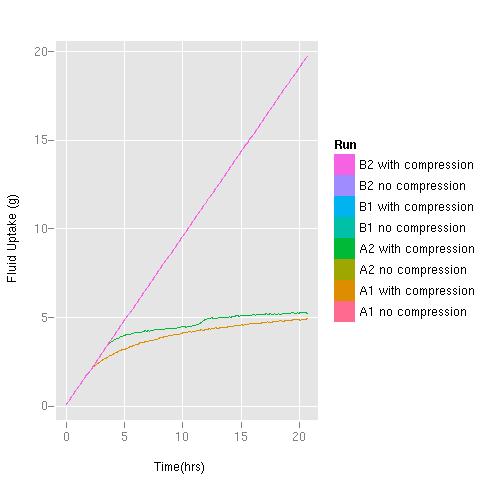
Note: the straight pink line hides a number of data sets superimposed on each other because the absorption rate of each dressing was greater than the fluid delivery rate: B2 with and without compression, B1 with and without compression; A2 without compression and A1 without compression. In the presence of compression Product A's absorbency was reduced - see the green and orange lines (A2 with compression and A1 with compression)
In the clinical situation, when treating heavily exuding wounds such as venous leg ulcers, it may be appropriate to select a product for exudate management that has been shown to perform well when subjected to clinically relevant levels of compression.
When measuring or comparing the absorbent capacity of dressings in the laboratory, it is therefore essential to take account of the effects of compression if the results of the investigation are to have any clinical relevance. The test system described in the present study clearly facilitates such comparisons, and appears to be able to detect significant differences in performance under compression.
This investigation was designed purely to assess the effect of compression on absorbance. Evaporative loss was prevented by the occlusive nature of the weighted pressure plate and this would undoubtedly have reduced the total fluid handling capacity of both products. This parameter can be accounted for by a modification to the test equipment if required, which is the addition of a chamber filled with silica gel, used to simulate a humidity gradient across the dressing.
This article has been made possible through an unrestricted educational grant from Coloplast www.coloplast.com.
1. World Union of Wound Healing Societies (WUWHS). Principles of Best Practice: Wound Exudate and the role of dressings: A consenus documument. London: MEP Ltd, 2007.
2. Thomas S. Functions of a wound dressing. In: Wound Management and Dressings. London: Pharmaceutical Press, 1990.
3. Thomas S, Fram P. The development of a novel technique for predicting the exudate handling properties of modern wound dressings. World Wide Wounds 2001; available from URL: http://www.worldwidewounds.com/2001/december/Thomas/absorbency-wound-dressings.html.
4. Grocott P. Opinion (description of WRAP project). HES (Healthcare Equipment and Supplies) February 8, 2006.
5. Lamke LO, Nilsson GE, Reithner HL. The evaporative water loss from burns and water vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977; 3: 159-65.
6. Thomas S, Fear M, Humphreys J, Disley L, Waring MJ. The effect of dressings on the production of exudate from venous leg ulcers. Wounds 1996; 8(5): 145-50.
7. Backhouse CM, Blair SD, Savage AP, Walton J, McCollum CN. Controlled trial of occlusive dressings in healing chronic venous ulcers. Br J Surg 1987; 74(7): 626-7.